N-甲基二乙酰胺 | 1113-68-4
中文名称
N-甲基二乙酰胺
中文别名
——
英文名称
N-methyldiacetamide
英文别名
N-diacetylmethylamine;DAMA;N-acetyl-N-methylacetamide;N,N-diacetyl(methyl)amine;N-Methyl-diacetamid;Diacetyl-methyl-amin
CAS
1113-68-4
化学式
C5H9NO2
mdl
MFCD00014968
分子量
115.132
InChiKey
ZNQFZPCFVNOXJQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-25°C
-
沸点:192-194°C
-
密度:1,06 g/cm3
-
闪点:192-194°C
-
稳定性/保质期:
在常温常压下保持稳定,应避免与强氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):-0.5
-
重原子数:8
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:37.4
-
氢给体数:0
-
氢受体数:2
安全信息
-
TSCA:Yes
-
危险品标志:Xn,Xi,T
-
安全说明:S26,S36,S36/37/39,S39
-
危险类别码:R20/21/22,R36/37/38,R22,R37/38,R41
-
WGK Germany:3
-
海关编码:2925190090
-
包装等级:III
-
危险类别:6.1
-
危险品运输编号:UN 3276 6.1/PG 3
-
储存条件:请将容器密封保存,并存放在阴凉、干燥处。
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product identifiers
Product name : N-Acetyl-N-methylacetamide
CAS-No. : 1113-68-4
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008 [EU-GHS/CLP]
Eye irritation (Category 2)
This substance is not classified as dangerous according to Directive 67/548/EEC.
Label elements
Labelling according Regulation (EC) No 1272/2008 [CLP]
Pictogram
Signal word Warning
Hazard statement(s)
H319 Causes serious eye irritation.
Precautionary statement(s)
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove
contact lenses, if present and easy to do. Continue rinsing.
Supplemental Hazard none
Statements
Other hazards - none
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Formula : C5H9NO2
Molecular Weight : 115,13 g/mol
Section 4. FIRST AID MEASURES
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Consult a physician.
In case of eye contact
Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.
Most important symptoms and effects, both acute and delayed
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Indication of any immediate medical attention and special treatment needed
no data available
Section 5. FIREFIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides, nitrogen oxides (NOx)
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid breathing vapors, mist or gas. Ensure adequate ventilation.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Soak up with inert absorbent material and dispose of as hazardous waste. Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Avoid contact with skin and eyes. Avoid inhalation of vapour or mist.
Normal measures for preventive fire protection.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Containers which are
opened must be carefully resealed and kept upright to prevent leakage.
Specific end uses
no data available
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and
at the end of workday.
Personal protective equipment
Eye/face protection
Safety glasses with side-shields conforming to EN166 Use equipment for eye protection tested
and approved under appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
impervious clothing, The type of protective equipment must be selected according to the
concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Where risk assessment shows air-purifying respirators are appropriate use a full-face respirator
with multi-purpose combination (US) or type ABEK (EN 14387) respirator cartridges as a backup
to engineering controls. If the respirator is the sole means of protection, use a full-face supplied air
respirator. Use respirators and components tested and approved under appropriate government
standards such as NIOSH (US) or CEN (EU).
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
a) Appearance Form: liquid
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing Melting point/range: -25 °C
point
f) Initial boiling point and 195 °C at 1.013 hPa
boiling range
g) Flash point no data available
h) Evaporation rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density 1,066 g/cm3
n) Water solubility no data available
o) Partition coefficient: n- log Pow: 0,05
octanol/water
p) Autoignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
Section 10. STABILITY AND REACTIVITY
Reactivity
no data available
Chemical stability
no data available
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
Section 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Potential health effects
Inhalation May be harmful if inhaled. May cause respiratory tract irritation.
Ingestion May be harmful if swallowed.
Skin May be harmful if absorbed through skin. May cause skin irritation.
Eyes
Causes eye irritation.
Signs and Symptoms of Exposure
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Additional Information
RTECS: Not available
Section 12. ECOLOGICAL INFORMATION
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
no data available
Other adverse effects
no data available
Section 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed
professional waste disposal service to dispose of this material.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
SECTION 15 - REGULATORY INFORMATION
N/A
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N,N-二甲基乙酰胺 N,N-dimethyl acetamide 127-19-5 C4H9NO 87.1216
反应信息
-
作为反应物:描述:参考文献:名称:Stereodynamics of diethylmethylamine and triethylamine摘要:DOI:10.1021/ja00387a012
-
作为产物:描述:参考文献:名称:烯酮与甲硅烷基化胺或酰胺的相互作用摘要:在对乙烯酮与单或二烷基氨基硅烷的相互作用的研究中,观察到许多新的重排。当受到烯酮作用时,二烷基氨基硅烷根据所施加的条件,会生成β-甲硅烷氧基乙烯基乙酸(I)的酰胺(路线“ a”,方案A)或甲硅烷基乙酸的酰胺(II)(路线“ b”,“ C”)。在两种情况下,均分离出中间体α-甲硅烷氧基乙烯基二烷基胺(III),可以将其重新排列以得到酰胺(II)。DOI:10.1016/s0022-328x(00)88613-1
-
作为试剂:描述:methyl (2S,4S,5R,6R)-5-acetamido-2,4-diacetyloxy-6-[(1R,2S)-1,2,3-triacetyloxypropyl]oxane-2-carboxylate 在 水 、 sodium 、 potassium carbonate 、 N-甲基二乙酰胺 、 sodium hydroxide 作用下, 以 甲醇 、 氯仿 、 水 、 乙腈 为溶剂, 反应 195.0h, 生成 (2R,4S,5R,6R)-5-acetamido-4-hydroxy-6-[(1R,2S)-1,2,3-trihydroxypropyl]-2-{2-[4,7,10-tris(carboxymethyl)-1,4,7,10-tetraazacyclododecan-1-yl]ethoxy}oxane-2-carboxylic acid参考文献:名称:顺磁性唾液酸缀合物作为磁共振应用探针的合成与表征。摘要:作为临床诊断工具,使用顺磁性系统作为造影剂的磁共振成像(MRI)受到越来越多的关注。同时,顺磁性系统的NMR也可以应用于生化领域。例如,顺磁弛豫增强(PRE)的使用可以使结构细化,并可以分析涉及大分子复合物形成的瞬态动力学过程。在这里,我们报告了一种新的类似DOTA的唾液酸共轭物的合成和计算表征,当与合适的顺磁性金属配合使用时,可以在MRI和PRE应用中使用。DOI:10.1016/j.carres.2012.03.002
文献信息
-
Mid- and near-IR study of the hydrogen bond interaction between N-methyldiacetamide and phenols作者:N. Leroux、C. Samyn、Th. Zeegers-HuyskensDOI:10.1016/s0022-2860(98)00352-4日期:1998.7The hydrogen bond complexes between phenol derivatives (p K a = 10.20−8.20) and N -methyldiacetamide (NMD) are studied by mid- and near-IR spectroscopy in carbon tetrachloride solution. The equilibrium constants and enthalpies of complex formation are determined and the results suggest that the proton acceptor ability of NMD is lower than that of monocarbonyl bases. Complex formation results in decoupling
-
Acyl Iodides in Organic Synthesis. Reaction of Acyl Iodides with N,N-Dimethyl Carboxylic Acid Amides作者:M. G. Voronkov、I. P. Tsyrendorzhieva、V. I. RakhlinDOI:10.1134/s1070428010100064日期:2010.10Acyl iodides RCOI (R = Me, Ph) reacted with N,N-dimethylformamide and N,N-dimethylacetamide Me2NC(=O)R’ (R’ = H, Me) along two concurrent pathways involving transacylation and cleavage of the Me-N bond. The first pathway leads to the formation of acyl group exchange products, and the second, to the corresponding imides R’CON(Me)COR.
-
Reaction of iodo(trimethyl)silane with N,N-dimethyl carboxylic acid amides作者:M. G. Voronkov、I. P. Tsyrendorzhieva、A. V. Lis、V. I. RakhlinDOI:10.1134/s1070428010060035日期:2010.6(R = H, Me) at a molar ratio of 1: 2 involved mainly cleavage of the N-C(=O) bond with formation of up to 80% of N,N-dimethyltrimethylsilylamine Me3SiNMe2 and the corresponding acyl iodide RCOI. In the reaction with N,N-dimethylformamide, formyl iodide HCOI was detected for the first time by gas chromatography-mass spectrometry. The contribution of Me-N bond cleavage, leading to N-methyl-N-trimethylsilyl碘代(三甲基)硅烷与N,N-二甲基甲酰胺和N,N-二甲基乙酰胺Me 2 NCOR(R = H,Me)的摩尔比为1:2的反应主要涉及NC(= O)键的裂解形成高达80%的N,N-二甲基三甲基甲硅烷基胺Me 3 SiNMe 2和相应的酰基碘RCOI。在与N,N-二甲基甲酰胺的反应中,通过气相色谱-质谱法首次检测到甲酰碘HCOI。Me-N键断裂的贡献,导致N-甲基-N-三甲基甲硅烷基衍生物Me(Me 3Si)NCOR和碘甲烷要小得多。另一副产物是相应的N-甲基酰亚胺MeN(COR)2,其通过初始酰胺与酰基碘的反应形成。碘代(三甲基)硅烷与DMF和DMA反应的主要中间体是季铵盐[Me 2(Me 3 Si)N + COR] I- ,它通过N-CO和N-Me键的解离而分解。
-
Hofmann, Chemische Berichte, 1881, vol. 14, p. 2730作者:HofmannDOI:——日期:——
-
Difficulties in the interaction of the free electron pair in diacetophosphides and diacetamides作者:R. G. Kostyanovskii、V. V. Yakshin、S. L. Zimont、I. I. ChervinDOI:10.1007/bf00908447日期:1968.2
表征谱图
-
氢谱1HNMR
-
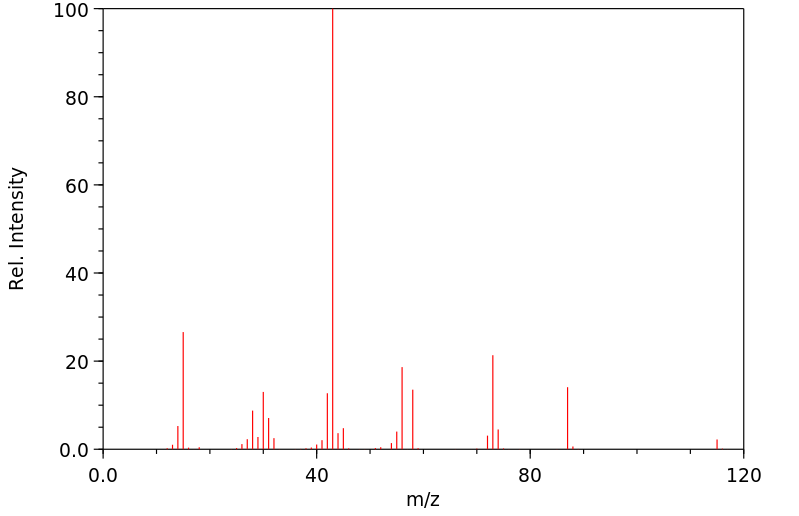
质谱MS
-
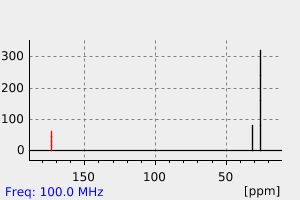
碳谱13CNMR
-
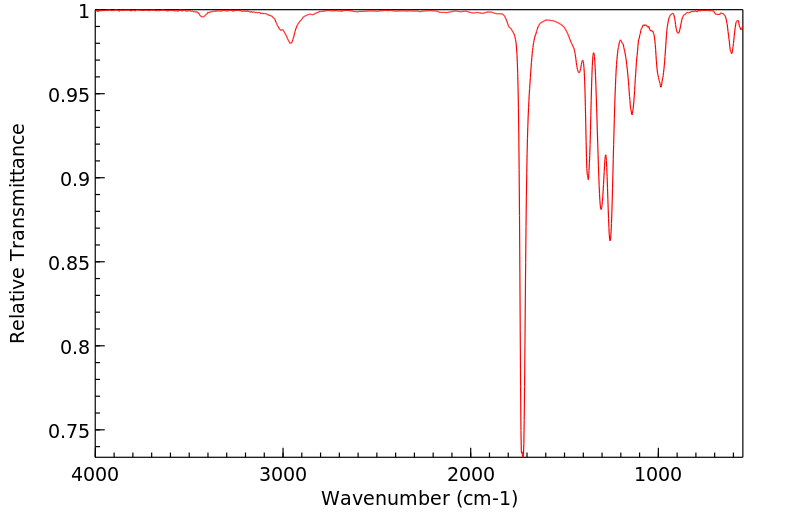
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸