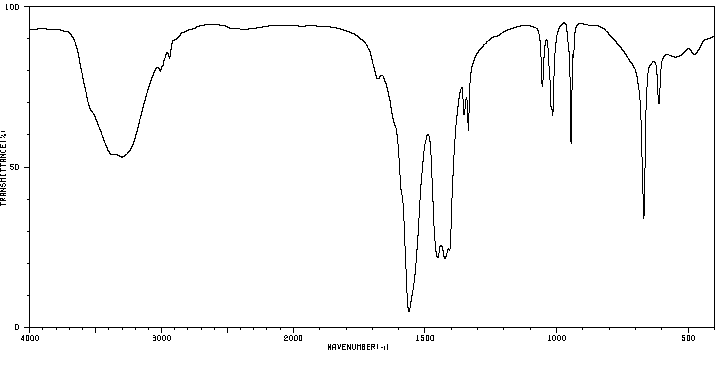
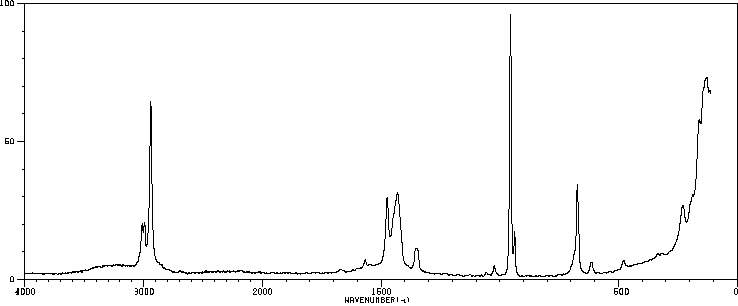
毒理性
纤维原性 - 引发组织损伤和纤维化(疤痕形成)。
Fibrogenic - Inducing tissue injury and fibrosis (scarring).
来源:Haz-Map, Information on Hazardous Chemicals and Occupational Diseases