Lucigenin | 2315-97-1
中文名称
——
中文别名
——
英文名称
Lucigenin
英文别名
10-methyl-9-(10-methylacridin-10-ium-9-yl)acridin-10-ium;dinitrate
CAS
2315-97-1
化学式
C28H22N4O6
mdl
MFCD00011925
分子量
510.5
InChiKey
KNJDBYZZKAZQNG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:250°C
-
溶解度:在醋酸中的溶解度为10mg/mL
-
最大波长(λmax):455nm
计算性质
-
辛醇/水分配系数(LogP):-2.63
-
重原子数:38
-
可旋转键数:1
-
环数:6.0
-
sp3杂化的碳原子比例:0.071
-
拓扑面积:134
-
氢给体数:0
-
氢受体数:6
安全信息
-
危险等级:5.1
-
安全说明:S24/25
-
WGK Germany:3
-
海关编码:29339900
SDS
制备方法与用途
反应信息
-
作为产物:参考文献:名称:SHEN, JIANMIN;SUN, TIEMIN;CHEN, RONGLIANG;XU, XIAOQING, XUASYUEH SHITSZI, 10,(1988) N 3, S. 178-179摘要:DOI:
文献信息
-
[EN] METHOD<br/>[FR] MÉTHODE申请人:UNIV OSLO HF公开号:WO2019243757A1公开(公告)日:2019-12-26The invention provides mitochondria-targeted chemiluminescent agents and their use in methods of photodynamic therapy (PDT). In particular, the invention provides compounds of general formula (I), and their pharmaceutically acceptable salts: (I) in which A represents a chemiluminescent moiety; each L, which may be the same or different, is either a direct bond or a linker; each B, which may be the same or different, represents a mitotropic moiety; n is an integer from 1 to 3, preferably 1; and x is an integer from 1 to 3, preferably 1. Such compounds find particular use in the treatment of deeply- sited tumours, e.g. glioblastoma multiforme (GBM), when used in combination with a photosensitizer or photosensitizer precursor.
-
[EN] INHIBITORS OF HUMAN IMMUNODEFICIENCY VIRUS REPLICATION<br/>[FR] INHIBITEURS DE LA RÉPLICATION DU VIRUS DE L'IMMUNODÉFICIENCE HUMAINE申请人:BOEHRINGER INGELHEIM INT公开号:WO2009062289A1公开(公告)日:2009-05-22Compounds of formula I : wherein c, R2, R3, R4, R5, R6, R7 and R8 are defined herein, are useful as inhibitors of HIV replication.公式I的化合物:其中c、R2、R3、R4、R5、R6、R7和R8按本说明定义,可用作HIV复制的抑制剂。
-
[EN] NOVEL BIPHENYL SARTANS<br/>[FR] NOUVEAUX BIPHÉNYLSARTANS申请人:UNIV MELBOURNE公开号:WO2011134019A1公开(公告)日:2011-11-03The present invention relates to compounds and compositions useful in modulating the renin-angiotensin system (RAS) in cells, and in particular to compounds which, inter alia, act as angiotensin II antagonists by binding to angiotensin II receptors. The invention also relates to the use of these compounds and compositions in the treatment of conditions responsive to angiotensin II antagonists such as hypertension, edema, renal failure, benign prostatic hypertrophy glaucoma, atherosclerosis, diabetes, Alzheimer's disease and congestive heart failure.
-
A method of using synthetic L-SE-Methylselenocysteine as a nutriceutical and a method of its synthesis申请人:PharmaSe, Incorporated公开号:EP1205471A1公开(公告)日:2002-05-15A synthesis of and use of L-Se-methylselenocysteine as a nutriceutical is described, based upon the knowledge that L-Se-methylselenocysteine is less toxic than L-selenomethionine towards normal cells. The synthesis proceeds by mixing N-(tert-butoxycarbonyl)-L-serine with a dialkyl diazodicarboxylate and at least one of a trialkylphosphine, triarylphosphine and phosphite to form a first mixture that includes N-(tert-butoxycarbonyl)-L-serine β-lactone. Methyl selenol or its salt is mixed with the N-(tert-butoxycarbonyl)-L-serine β-lactone to form a second mixture that includes N-(tert-butoxycarbonyl)-Se-methylselenocysteine. The text butoxycarbonyl group is removed from the N-(tert-butoxycarbonyl)-Se-methylselenocysteine to form L-Se-methylselenocysteine. This synthesis significantly improves the manufacturability, manufacturing efficiency and utility of this naturally occurring rare form of organic-selenium. L-Se-methylselenocysteine formed, for example, in this manner may be used as a nutriceutical for supplementation into the diets of humans or animals for various beneficial purposes, such as, for example, to prevent or reduce the risk of developing cancer.描述了一种合成和使用L-Se-甲硒氨酸作为一种营养保健品,基于L-Se-甲硒氨酸对正常细胞比L-硒蛋氨酸毒性较小的知识。合成过程包括将N-(叔丁氧羰基)-L-丝氨酸与二烷基重氮二羧酸酯和三烷基膦、三芳基膦和磷酸酯中的至少一种混合,形成包含N-(叔丁氧羰基)-L-丝氨酸β-内酯的第一混合物。甲硒醇或其盐与N-(叔丁氧羰基)-L-丝氨酸β-内酯混合,形成包含N-(叔丁氧羰基)-Se-甲硒氨酸的第二混合物。将N-(叔丁氧羰基)-Se-甲硒氨酸中的叔丁氧羰基团去除,形成L-Se-甲硒氨酸。这种合成显著改善了这种天然存在的稀有有机硒形式的可制造性、制造效率和实用性。例如,以这种方式形成的L-Se-甲硒氨酸可用作一种营养保健品,添加到人类或动物的饮食中,以达到各种有益目的,例如预防或减少患癌症的风险。
-
Reagents and methods for direct labeling of nucleotides申请人:Naleway John J.公开号:US20130150254A1公开(公告)日:2013-06-13The present invention provides systems and methods for production of activatable diazo-derivatives for use in labeling nucleotides. Labeling nucleotides is accomplished by contacting a stable hydrazide derivative of a detectable moiety with an activating polymer reagent which is used to directly label the nucleotide sample. Labeling occurs on the phosphate backbone of the nucleotide which does not perturb hybridization of the labeled nucleotide with its anti-sense strand. Since the method involves direct labeling, all types of nucleotides can be labeled without prior amplification or alteration.
表征谱图
-
氢谱1HNMR
-
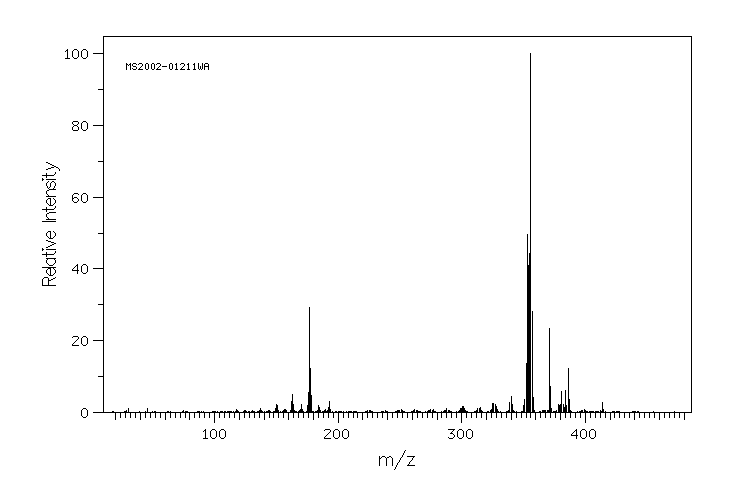
质谱MS
-
碳谱13CNMR
-
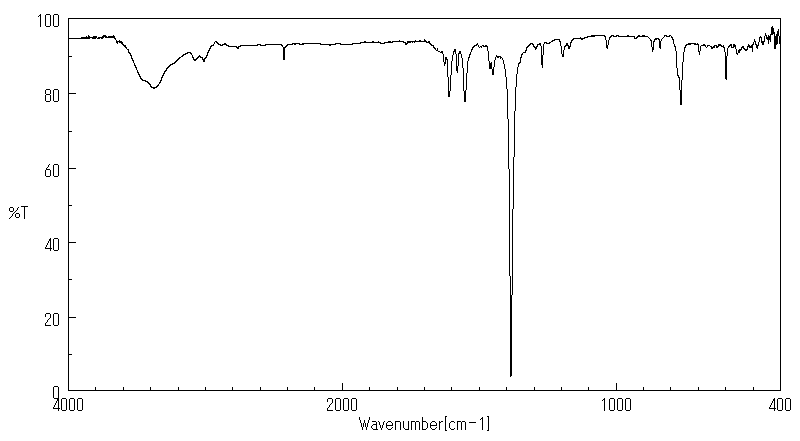
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-(叔丁基)-2-(喹啉-2-基)-4,5-二氢噁唑
(SP-4-1)-二氯双(喹啉)-钯
(E)-2-氰基-3-[5-(2,5-二氯苯基)呋喃-2-基]-N-喹啉-8-基丙-2-烯酰胺
(8α,9S)-(+)-9-氨基-七氢呋喃-6''-醇,值90%
(6,7-二甲氧基-4-(3,4,5-三甲氧基苯基)喹啉)
(1-羟基-5-硝基-8-氧代-8,8-dihydroquinolinium)
黄尿酸 8-甲基醚
麻保沙星EP杂质D
麻保沙星EP杂质B
麻保沙星EP杂质A
麦角腈甲磺酸盐
麦角腈
麦角灵
麦皮星酮
麦特氧特
高铁试剂
高氯酸3-苯基[1,3]噻唑并[3,2-f]5-氮杂菲-4-正离子
马波沙星EP杂质F
马波沙星
马来酸茚达特罗杂质
马来酸茚达特罗
马来酸维吖啶
马来酸来那替尼
马来酸四甲基铵
香草木宁碱
颜料红R-122
颜料红210
颜料红
顺式-苯并(f)喹啉-7,8-二醇-9,10-环氧化物
顺式-(alphaR)-N-(4-氯苯基)-4-(6-氟-4-喹啉基)-alpha-甲基环己烷乙酰胺
非那沙星
非那沙星
青花椒碱
青色素863
雷西莫特
隐花青
阿莫地喹-d10
阿莫地喹
阿莫吡喹N-氧化物
阿美帕利
阿米诺喹
阿立哌唑溴代杂质
阿立哌唑杂质B
阿立哌唑杂质38
阿立哌唑杂质1750
阿立哌唑杂质13
阿立哌唑杂质
阿立哌唑杂质
阿尔马尔
阿加曲班杂质43