三异丙叉丙酮基硼烷 | 7297-95-2
中文名称
三异丙叉丙酮基硼烷
中文别名
——
英文名称
trimesitylborane
英文别名
Trimesityl-boran;tris(2,4,6-trimethylphenyl)borane
CAS
7297-95-2
化学式
C27H33B
mdl
MFCD00008539
分子量
368.37
InChiKey
FXFWLXFJBVILBE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:193-195 °C (lit.)
-
沸点:477.6±45.0 °C(Predicted)
-
密度:0.98±0.1 g/cm3(Predicted)
-
稳定性/保质期:
在常温常压下,该物质是稳定的。
计算性质
-
辛醇/水分配系数(LogP):10.11
-
重原子数:28
-
可旋转键数:3
-
环数:3.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
WGK Germany:3
-
安全说明:S22,S24,S25
-
储存条件:请将药品存放在密闭、阴凉干燥的地方。
SDS
SECTION 1: Identification of the substance/mixture and of the company/undertaking
Product identifiers
Product name : Trimesitylborane
REACH No. : A registration number is not available for this substance as the substance
or its uses are exempted from registration, the annual tonnage does not
require a registration or the registration is envisaged for a later
registration deadline.
CAS-No. : 7297-95-2
SECTION 2: Hazards identification
Classification of the substance or mixture
Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008.
This substance is not classified as dangerous according to Directive 67/548/EEC.
Label elements
Other hazards - none
SECTION 3: Composition/information on ingredients
Substances
Formula : C27H33B
Molecular Weight : 368,36 g/mol
CAS-No. : 7297-95-2
No components need to be disclosed according to the applicable regulations.
SECTION 4: First aid measures
Description of first aid measures
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration.
In case of skin contact
Wash off with soap and plenty of water.
In case of eye contact
Flush eyes with water as a precaution.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water.
Most important symptoms and effects, both acute and delayed
The most important known symptoms and effects are described in the labelling (see section 2.2) and/or in
section 11
Indication of any immediate medical attention and special treatment needed
no data available
SECTION 5: Firefighting measures
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides, Borane/boron oxides
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
SECTION 6: Accidental release measures
Personal precautions, protective equipment and emergency procedures
Avoid dust formation. Avoid breathing vapours, mist or gas.
For personal protection see section 8.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Sweep up and shovel. Keep in suitable, closed containers for disposal.
Reference to other sections
For disposal see section 13.
SECTION 7: Handling and storage
Precautions for safe handling
Provide appropriate exhaust ventilation at places where dust is formed.Normal measures for preventive fire
protection.
For precautions see section 2.2.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Recommended storage temperature: 2 - 8 °C
Specific end use(s)
A part from the uses mentioned in section 1.2 no other specific uses are stipulated
SECTION 8: Exposure controls/personal protection
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
General industrial hygiene practice.
Personal protective equipment
Eye/face protection
Use equipment for eye protection tested and approved under appropriate government standards
such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
Choose body protection in relation to its type, to the concentration and amount of dangerous
substances, and to the specific work-place., The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Respiratory protection is not required. Where protection from nuisance levels of dusts are desired,
use type N95 (US) or type P1 (EN 143) dust masks. Use respirators and components tested and
approved under appropriate government standards such as NIOSH (US) or CEN (EU).
Control of environmental exposure
Do not let product enter drains.
SECTION 9: Physical and chemical properties
Information on basic physical and chemical properties
a) Appearance Form: solid
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing Melting point/range: 193 - 195 °C
point
f) Initial boiling point and no data available
boiling range
g) Flash point no data available
h) Evapouration rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility no data available
o) Partition coefficient: n- no data available
octanol/water
p) Auto-ignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
SECTION 10: Stability and reactivity
Reactivity
no data available
Chemical stability
Stable under recommended storage conditions.
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
In the event of fire: see section 5
SECTION 11: Toxicological information
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitisation
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Additional Information
RTECS: Not available
SECTION 12: Ecological information
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
PBT/vPvB assessment not available as chemical safety assessment not required/not conducted
Other adverse effects
no data available
SECTION 13: Disposal considerations
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company.
Contaminated packaging
Dispose of as unused product.
SECTION 14: Transport information
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
SECTION 15: Regulatory information
This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.
Safety, health and environmental regulations/legislation specific for the substance or mixture
no data available
Chemical Safety Assessment
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 氯双(2,4,6-三甲基苯基)硼烷 dimesityl(chloro)borane 240432-83-1 C18H22BCl 284.637 二(三甲苯基)氟化硼 dimesitylfluoroborane 436-59-9 C18H22BF 268.182
反应信息
-
作为反应物:参考文献:名称:Lewis酸性硼烷活化无金属氢的化学反应新模式。摘要:我们在此探索三(芳基)硼烷路易斯酸是否能够在沮丧的路易斯对(FLP)的概念所描述的通常的路易斯酸/碱化学之外裂解H 2。代替路易斯碱,我们使用化学还原剂生成两个高度受阻的硼烷的稳定自由基阴离子:三(3,5-二硝基甲硅烷基)硼烷和三(甲硅烷基)硼烷。NMR光谱表征揭示了相应的硼烷自由基阴离子激活(裂解)二氢,而EPR光谱表征在计算分析的支持下揭示了沿氢活化途径的中间体。与传统的FLP化学模型(称为杂化裂解途径)相比,这种基于自由基的氧化还原途径涉及H2的均质裂解。DOI:10.1002/anie.201900861
-
作为产物:参考文献:名称:Lewis酸性硼烷活化无金属氢的化学反应新模式。摘要:我们在此探索三(芳基)硼烷路易斯酸是否能够在沮丧的路易斯对(FLP)的概念所描述的通常的路易斯酸/碱化学之外裂解H 2。代替路易斯碱,我们使用化学还原剂生成两个高度受阻的硼烷的稳定自由基阴离子:三(3,5-二硝基甲硅烷基)硼烷和三(甲硅烷基)硼烷。NMR光谱表征揭示了相应的硼烷自由基阴离子激活(裂解)二氢,而EPR光谱表征在计算分析的支持下揭示了沿氢活化途径的中间体。与传统的FLP化学模型(称为杂化裂解途径)相比,这种基于自由基的氧化还原途径涉及H2的均质裂解。DOI:10.1002/anie.201900861
-
作为试剂:描述:(R)-3-(tert-butyldiphenylsilyloxy)-2-methylpropan-1-ol 在 草酰氯 、 三异丙叉丙酮基硼烷 、 potassium tert-butylate 、 叔丁基锂 、 sodium 、 二异丁基氢化铝 、 二甲基亚砜 作用下, 以 四氢呋喃 、 乙醚 、 正己烷 、 二氯甲烷 为溶剂, 反应 42.17h, 生成 (1S,6S)-(+)-4-[(4R,E)-5-tert-butyldiphenylsilyloxy-4-methyl-2-pentenyloxy]-1,5-dimethyl-6-hydroxy-9-oxo-8-oxabicyclo[4.3.0]non-4(5)-ene参考文献:名称:研究以全合成和确定 (-)-saudin 的绝对构型而告终摘要:对最终对两种对映体的全合成及其绝对构型分配的研究的完整说明,这是一种降血糖的天然产物。描述了两种方法,第一种通过双环内酯中间体和相关的第二种单环酯进行。前者通过不对称 Diels-Alder 环加成获得,后者通过不对称环化方案获得。两种方法都采用路易斯酸促进的克莱森重排,成功的方法利用双齿螯合来控制关键克莱森重排的面部选择性。DOI:10.1016/j.tet.2011.09.067
文献信息
-
Lewis Acidic Boranes, Lewis Bases, and Equilibrium Constants: A Reliable Scaffold for a Quantitative Lewis Acidity/Basicity Scale作者:Robert J. Mayer、Nathalie Hampel、Armin R. OfialDOI:10.1002/chem.202003916日期:2021.2.24borane/Lewis base combination through the sum of two descriptors, one for Lewis acidity (LAB) and one for Lewis basicity (LBB). The resulting Lewis acidity/basicity scale is independent of fixed reference acids/bases and valid for various types of trivalent boron‐centered Lewis acids. It is demonstrated that the newly developed Lewis acidity/basicity scale is easily extendable through linear relationships with基于三芳基硼烷与各种以 O、N、S 和 P 为中心的路易斯碱在二氯甲烷中反应的 90 个实验平衡常数,开发了针对以硼为中心的路易斯酸的定量路易斯酸度/碱度标度。 20℃。使用线性自由能关系 log K B = LA B + LB B进行分析,可以通过两个描述符(一个用于路易斯酸度 ( LA B ))的总和来计算任何类型的硼烷/路易斯碱组合的平衡常数K B。一个用于路易斯碱度(LB B)。所得的路易斯酸度/碱度标度与固定的参考酸/碱无关,并且对于各种类型的三价硼中心路易斯酸有效。事实证明,新开发的路易斯酸度/碱度标度可以通过与量子化学计算或常见的物理有机描述符和已知热力学数据(Δ H )的线性关系轻松扩展。此外,该实验平台可用于硼烷催化反应的合理发展。
-
四取代吡咯并[3,2-b]吡咯的衍生物和基于其的有机电致发光器件
-
Skeletal rearrangements of arylborane complexes mediated by redox reactions: thermal and photochemical oxidation by metal ions作者:John J. Eisch、Jamshed H. Shah、Marek P. BoleslawskiDOI:10.1016/0022-328x(94)87003-9日期:1994.1variety of metal salts have been found to undergo reduction by thermal and photochemical interaction with tetraarylborate salts and with neutral alkyl- and aryl-borane complexes. In the cases of Cu2+, Cu+, Ni2+, Co2+, Pd2+, Pt2+, Ag+, Zn2+, Hg2+, Sn2+, Pb2+ and Rh3+ salts, such photochemical reductions with NaBPh4 led to the deposition of the free metal, while a number of binary mixtures of metal salts已经发现各种金属盐通过与四芳基硼酸盐以及中性烷基-和芳基-硼烷配合物的热和光化学相互作用而发生还原。在Cu 2 +,Cu +,Ni 2 +,Co 2 +,Pd 2 +,Pt 2 +,Ag +,Zn 2 +,Hg 2 +,Sn 2 +,Pb 2+和Rh 3+盐的情况下,例如用NaBPh 4进行光化学还原在这种光还原作用下,导致了游离金属的沉积,而许多金属盐的二元混合物导致了两种金属(有时是真正的合金)的沉积。在这些减少的过程中,芳基硼酸酯的还原剂后行氧化芳基的偶联以形成联芳基严格帧内离子的(BAR 4 - )或分子内(的Ar 3B)分别方式。对四芳基硼酸根阴离子本身,四苯基硼酸亚铜和三苯基硼烷-吡啶配合物的光化学的单独研究已提出了能够用作金属离子光还原剂的各种反应性中间体的证据,例如三芳基硼烷自由基阴离子,二芳基硼酸酯(I)阴离子或芳基硼烷,7-硼环环庚二烯阴离子或中性络合物,最后是芳基硼氢
-
A comparative study of base-free arylcopper reagents for the transfer of aryl groups to boron halides作者:Anand Sundararaman、Frieder JäkleDOI:10.1016/s0022-328x(03)00588-6日期:2003.9A comparative study on the reactivity and selectivity of arylcopper species in reactions with boron halides was performed. Mesitylcopper reacts with BX3 (X=Cl, Br) in toluene at low temperature under highly selective formation of the monosubstituted boranes MesBX2. The dimesitylboranes Mes2BX are gradually formed with a twofold excess of the organocopper reagent at elevated temperature. In contrast对芳基铜与卤化硼反应的反应性和选择性进行了比较研究。Mesitylcopper在低温下与甲苯中的BX 3(X = Cl,Br)在高度选择性形成单取代硼烷MesBX 2的条件下反应。在升高的温度下,用两倍过量的有机铜试剂逐渐形成二聚三聚异戊二烯Mes 2 BX。相反,五氟苯基铜在与BX 3反应中显示出形成B(C 6 F 5)3的趋势。不管所用的化学计量如何,都表明电子因素对芳基转移反应的选择性有很大的影响。从碱合成五氟苯基硼卤化物C 6 F 5 BX 2(X = Cl:57%; X = Br:62%)以及三(五氟苯基)硼烷(80%)和相关的混合取代的三芳基硼烷的新方法无氟可分离的五氟苯基铜前体已被开发出来。
-
First structural characterization of a boron-centered radical: x-ray crystal structure of [Li(12-crown-4)2]+ [BMes3]-.bul.作者:Marilyn M. Olmstead、Philip P. PowerDOI:10.1021/ja00274a071日期:1986.7In this paper the authors present the first structural determination of a free (BAr/sub 3/)/sup -/ radical anion which was crystallized as the crown ether salt (Li(12-crown-4)/sub 2/)/sup +/(BMes/sub 3/)/sup -/. The crystal structure was determined to establish the existence of (BMes/sub 3/)/sup -/ as a free noninteraction radical anion. Although the structure of the precursor, BMes/sub 3/, was already
表征谱图
-
氢谱1HNMR
-
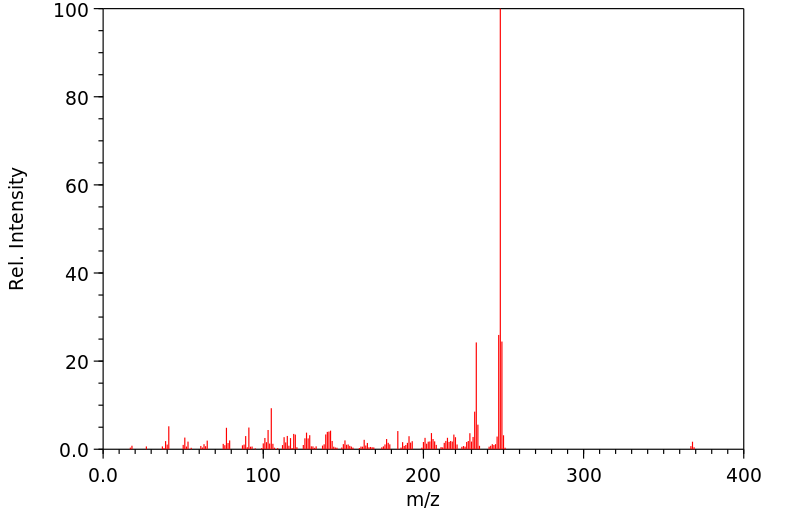
质谱MS
-
碳谱13CNMR
-
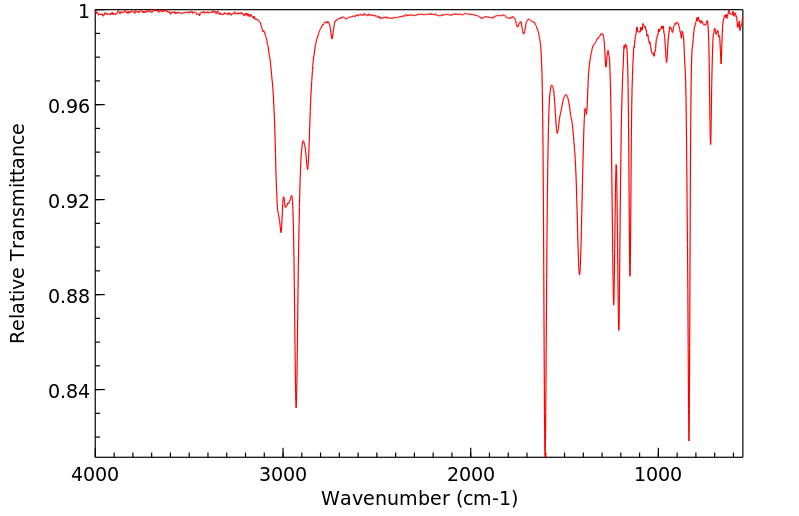
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫