乙基(2E)-2-氰基-3-(4-甲基苯基)丙烯酸酯 | 2017-88-1
中文名称
乙基(2E)-2-氰基-3-(4-甲基苯基)丙烯酸酯
中文别名
——
英文名称
ethyl (E)-2-cyano-3-p-tolylacrylate
英文别名
ethyl (E)-2-cyano-3-(4-methylphenyl)-2-propenoate;(E)-ethyl 2-cyano-3-p-tolylacrylate;2-Ethoxycarbonyl-3-(4-methylphenyl)-2-propenonitrile;Ethyl 2-cyano-3-(4-methylphenyl)acrylate;ethyl (E)-2-cyano-3-(4-methylphenyl)prop-2-enoate
CAS
2017-88-1
化学式
C13H13NO2
mdl
——
分子量
215.252
InChiKey
KKIZDEFOEGGSKX-XYOKQWHBSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:86-89°C
-
沸点:160-162 °C(Press: 4 Torr)
-
密度:1.116±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:16
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.23
-
拓扑面积:50.1
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
海关编码:2926909090
SDS
反应信息
-
作为反应物:描述:乙基(2E)-2-氰基-3-(4-甲基苯基)丙烯酸酯 在 盐酸 、 sodium hydride 作用下, 以 四氢呋喃 为溶剂, 反应 74.08h, 生成 3-(p-tolyl)pentanedioic acid参考文献:名称:Synthesis and binding affinities of methylvesamicol analogs for the acetylcholine transporter and sigma receptor摘要:We synthesized methylvesamicol analogs 13-16 and investigated the binding characteristics of 2-[4-phenylpiperidino]cyclohexanol (vesamicol) and methylvesamicol analogs 13-16, with a methyl group introduced into the 4-phenylpiperidine moiety, to sigma receptors (sigma-1, sigma-2) and to vesicular acetylcholine transporters (VAChT) in membranes of the rat brain and liver. In competitive inhibition studies, (-)-o-methylvesamicol [(-)-OMV] (13) (K-i = 6.7 nM), as well as (-)-vesamicol (K-i = 4.4 nM), had a high affinity for VAChT. (+)-p-Methylvesamicol [(+)-PMV] (16) (K-i = 3.0 nM), as well as SA4503 (K-i = 4.4 nM), reported as a sigma-1 mapping agent for positron emission tomography (PET), had a high affinity for the sigma-1 receptor. The binding affinity of (+)-PMV (1-16) for the sigma-1 receptor (K-i = 3.0 nM) was about 13 times higher than that for the sigma-2 (sigma-2) receptor (K-i = 40.7 nM). (+)-PMV (16) (K-i = 199 nM) had a much lower affinity for VAChT than SA4503 (K-i = 50.2 nM) and haloperidol (K-i = 41.4 nM). These results showed that the binding characteristics of (-)-OMV (13) to VAChT were similar to those of (-)-vesamicol and that (+)-PMV (16) bound to the sigma-1 receptor with high affinity. In conclusion, (-)-OMV (13) and (+)-PMV (16). which had a Suitable structure, with a methyl group for labeling with C-11, may become not only a new VAChT ligand and a new type of sigma receptor ligand, respectively, but may also become a new target compound of VAChT and the sigma-1 receptor radioligand for PET, respectively. (c) 2005 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmc.2005.11.044
-
作为产物:描述:参考文献:名称:Synthesis and binding affinities of methylvesamicol analogs for the acetylcholine transporter and sigma receptor摘要:We synthesized methylvesamicol analogs 13-16 and investigated the binding characteristics of 2-[4-phenylpiperidino]cyclohexanol (vesamicol) and methylvesamicol analogs 13-16, with a methyl group introduced into the 4-phenylpiperidine moiety, to sigma receptors (sigma-1, sigma-2) and to vesicular acetylcholine transporters (VAChT) in membranes of the rat brain and liver. In competitive inhibition studies, (-)-o-methylvesamicol [(-)-OMV] (13) (K-i = 6.7 nM), as well as (-)-vesamicol (K-i = 4.4 nM), had a high affinity for VAChT. (+)-p-Methylvesamicol [(+)-PMV] (16) (K-i = 3.0 nM), as well as SA4503 (K-i = 4.4 nM), reported as a sigma-1 mapping agent for positron emission tomography (PET), had a high affinity for the sigma-1 receptor. The binding affinity of (+)-PMV (1-16) for the sigma-1 receptor (K-i = 3.0 nM) was about 13 times higher than that for the sigma-2 (sigma-2) receptor (K-i = 40.7 nM). (+)-PMV (16) (K-i = 199 nM) had a much lower affinity for VAChT than SA4503 (K-i = 50.2 nM) and haloperidol (K-i = 41.4 nM). These results showed that the binding characteristics of (-)-OMV (13) to VAChT were similar to those of (-)-vesamicol and that (+)-PMV (16) bound to the sigma-1 receptor with high affinity. In conclusion, (-)-OMV (13) and (+)-PMV (16). which had a Suitable structure, with a methyl group for labeling with C-11, may become not only a new VAChT ligand and a new type of sigma receptor ligand, respectively, but may also become a new target compound of VAChT and the sigma-1 receptor radioligand for PET, respectively. (c) 2005 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmc.2005.11.044
文献信息
-
Phosphane-Catalyzed Knoevenagel Condensation: A Facile Synthesis ofα-Cyanoacrylates andα-Cyanoacrylonitriles作者:Jhillu S. Yadav、Basi V. Subba Reddy、Ashok K. Basak、Boddapati Visali、Akkirala Venkat Narsaiah、Kommu NagaiahDOI:10.1002/ejoc.200300513日期:2004.2Triphenylphosphane (TPP) has been utilized as a novel and efficient catalyst for the Knoevenagel condensation of aldehydes with acidic methylene compounds such as ethyl cyanoacetate and malononitrile to afford substituted olefins. The reaction proceeds smoothly under mild and solvent-free conditions and the products are obtained in excellent yields with an E-geometry. This method is applicable for
-
Synthesis and characterization of sulfamic acid supported on Fe<sub>3</sub> O<sub>4</sub> nanoparticles: A green, versatile and magnetically separable acidic catalyst for oxidation reactions and Knoevenagel condensation作者:Lotfi Shiri、Hojatollah Narimani、Mosstafa KazemiDOI:10.1002/aoc.3927日期:2018.1Sulfamic acid immobilized on diethylenetriamine functionalized Fe3O4 nanoparticles (SA‐DETA‐Fe3O4) was successfully prepared and characterized by X‐ray diffraction (XRD), Fourier transform infrared spectroscopy (FT‐IR), vibrating sample magnetometer (VSM), thermo gravimetric analysis (TGA), X‐Ray diffraction (XRD) and scanning electron microscopy (SEM). The sulfamic acid was found as a magnetically
-
A Simple, Efficient and Green Procedure for Knoevenagel Condensation in Water or under Solvent-free Conditions作者:Ya-Qin Yu、Zhong-Liang WangDOI:10.1002/jccs.201200391日期:2013.31,4‐Diazabicyclo[2.2.2]octane was used as an efficient catalyst in the Knoevenagel condensation reaction of various kinds of aromatic/aliphatic/heterocyclic/αβ‐unsaturated aldehydes and ketones with active methylene compounds. This is a convenient and rapid method for Knoevenagel condensation, which affords the corresponding substituted electrophilic alkenes in excellent yields. The reaction condition1,4-二氮杂双环[2.2.2]辛烷被用作各种芳族/脂肪族/杂环/αβ-不饱和醛和酮与活性亚甲基化合物的Knoevenagel缩合反应的有效催化剂。这是用于Knoevenagel缩合的一种方便快捷的方法,该方法以极高的收率提供了相应的取代的亲电子烯烃。反应条件温和并且该方法操作简单。仅检测到电子异构体的产物无需纯化。使用水作为反应介质使该方法对环境无害。催化剂可以循环使用六次而不会损失活性。
-
Transition metal polyhydrides-catalyzed addition of activated nitriles to aldehydes and ketones via Knoevenagel condensation作者:Yingrui Lin、Xianchao Zhu、Min XiangDOI:10.1016/0022-328x(93)80087-r日期:1993.4Transition metal polyhydrides and dihydrogen complexes catalyze Knoevenagel addition of cyanoacetate to aldehydes and ketones under neutral and mild conditions, the adducts undergo dehydration to give substituted (E)-2-cyano-α,β-unsaturated esters exclusively.
-
The Knoevenagel condensation using quinine as an organocatalyst under solvent-free conditions作者:Kavita Jain、Saikat Chaudhuri、Kuntal Pal、Kalpataru DasDOI:10.1039/c8nj04219e日期:——organocatalyst to afford various electrophilic alkenes in excellent yields (up to 90%). In the presence of a catalytic amount of quinine (15 mol%), the reaction proceeded at room temperature (RT) under solvent-free conditions. In this green approach, the organocatalyst was recovered and recycled for up to four cycles without appreciable loss of activity.
表征谱图
-
氢谱1HNMR
-
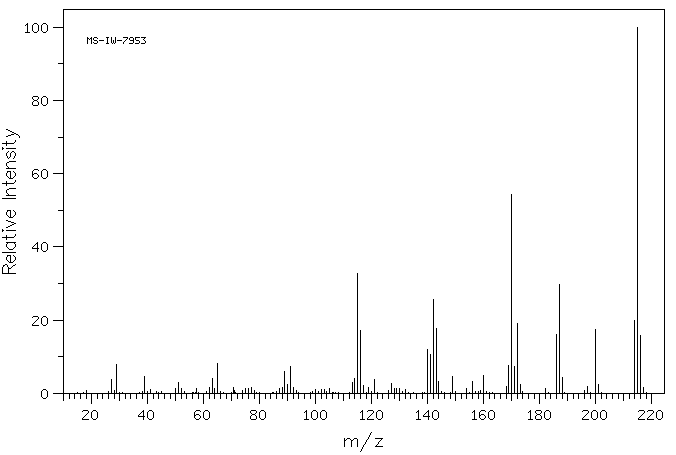
质谱MS
-
碳谱13CNMR
-
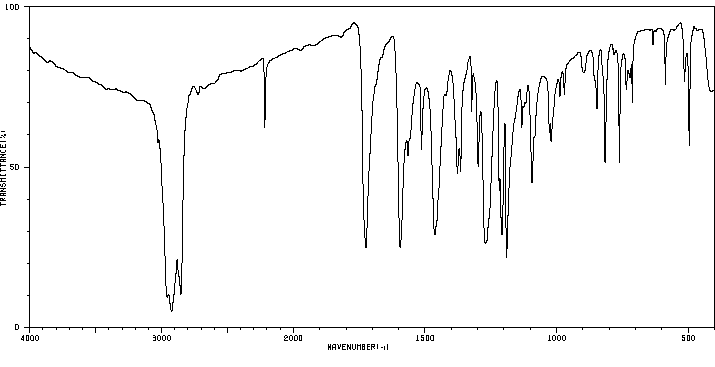
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(E)-3-(4-(叔丁基)苯基)丙烯酸乙酯
(E)-3-(2-(三氟甲基)苯基)丙烯酸乙酯
(E)-3-(2,4-二甲氧基苯基)丙烯酸乙酯
(2E)-N-[2-(3-羟基-2-氧代-2,3-二氢-1H-吲哚-3-基)乙基]-3-苯基丙-2-烯酰胺
黄金树苷
鲁索曲波帕
香豆酸肉桂酯
香豆酰多巴胺
香草醛缩丙酮
顺式邻羟基肉桂酸
顺式芥子酸
顺式-曲尼司特
顺式-乙基肉桂酸酯
顺式-N-阿魏酰酪胺
顺式-3,4-二甲氧基苯丙烯酸
顺式-2-((叔丁氧羰基)氨基)-3-(4-氨甲酰基-2,6-二甲苯基)丙烯酸甲酯
顺-o-羧基肉桂酸
顺-2-甲氧基肉桂酸
阿魏酸钠
阿魏酸酰胺
阿魏酸甲酯
阿魏酸甲酯
阿魏酸甲酯
阿魏酸松柏酯
阿魏酸杂质1
阿魏酸异辛酯
阿魏酸哌嗪
阿魏酸二十烷基酯
阿魏酸乙酯
阿魏酸4-O-硫酸二钠盐
阿魏酸-D3
阿魏酸
阿魏酸
阿魏酰酪胺
间羟基肉桂酸
间羟基肉桂酸
间硝基肉桂酸
间甲基肉桂酸
间甲基反式肉桂酸甲酯
间氯肉桂酸
间三氟甲氧基肉桂酸甲酯
间-香豆酸
间-(三氟甲基)-肉桂酸
锂(E)-2-溴-3-苯基丙烯酸酯
钠二乙基2-[(氧代氨基)-苯基亚甲基]丙二酸酯盐
酪氨酸磷酸化抑制剂AG 556
酪氨酸磷酸化抑制剂AG 527
酪氨酸磷酸化抑制剂AG 490
酪氨酸磷酸化抑制剂A46
酪氨酸磷酸化抑制剂 AG 30