乙醛苯腙 | 935-07-9
中文名称
乙醛苯腙
中文别名
——
英文名称
acetaldehyde phenylhydrazone
英文别名
Acetaldehyd-phenylhydrazon;N-(ethylideneamino)aniline
CAS
935-07-9
化学式
C8H10N2
mdl
——
分子量
134.181
InChiKey
KURBTRNHGDQKOS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:99.5°C
-
沸点:237.3°C (rough estimate)
-
密度:1.0698 (rough estimate)
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:10
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.12
-
拓扑面积:24.4
-
氢给体数:1
-
氢受体数:2
SDS
上下游信息
反应信息
-
作为反应物:描述:乙醛苯腙 在 lead(IV) acetate 、 溶剂黄146 作用下, 生成 2-(4-hydroxy-phenyl)-5-methyl-3-phenyl-tetrazolium; chloride参考文献:名称:Jerchel; Moehle, Chemische Berichte, 1944, vol. 77/79, p. 591,599摘要:DOI:
-
作为产物:参考文献:名称:Lockemann; Liesche, Justus Liebigs Annalen der Chemie, 1905, vol. 342, p. 27,28摘要:DOI:
文献信息
-
The use of enaminones and enamines as effective synthons for MSA-catalyzed regioselective synthesis of 1,3,4-tri- and 1,3,4,5-tetrasubstituted pyrazoles作者:Liancheng Duan、Hui Zhou、Yucheng Gu、Ping Gong、Mingze QinDOI:10.1039/c9nj03701b日期:——In the present work, an efficient regioselective synthesis of 1,3,4-tri- and 1,3,4,5-tetrasubstituted pyrazoles via a methanesulfonic acid (MSA)-catalyzed reaction of hydrazones with enaminones or enamines is reported. Mechanistically, the formation of the title compounds involves the [2+3] cycloaddition of hydrazones with enaminones or enamines followed by aromatization with acid and oxygen. This
-
Aryl Pyrazoles from Photocatalytic Cycloadditions of Arenediazonium作者:Luana Cardinale、Michael Neumeier、Michal Majek、Axel Jacobi von WangelinDOI:10.1021/acs.orglett.0c02514日期:2020.9.18conditions (rt, 20 min) with catalytic [Ru(bpy)3]2+ under blue-light irradiation and exhibited compatibility with several functional groups (e.g., I, SF5, SO2NH2, N3, CN) and perfect levels of regiocontrol. Mechanistic studies (luminescence spectroscopy, CV, DFT, radical trapping, quantum yield determination) documented an initial oxidative quenching of the excited photocatalyst and the operation of
-
Electrochemical Formation of Methoxy- and Cyano(phenylazo)alkanes from Aldehyde and Ketone Phenylhydrazones作者:Mitsuhiro Okimoto、Yukio Takahashi、Toyoji KakuchiDOI:10.1055/s-2003-41048日期:——Several aldehyde and ketone phenylhydrazones were electrooxidized in MeOH. Electrooxidation in the presence of KI or tetraethylammonium p-toluenesulfonate as the supporting electrolyte afforded the corresponding methoxy(phenylazo)alkanes. whereas electrooxidation in the presence of KI, NaCN, and HOAc afforded the cyano(phenylazo)alkanes.
-
Reduction of hydrazines to amines with aqueous solution of titanium(iii) trichloride作者:Yan Zhang、Qiang Tang、Meiming LuoDOI:10.1039/c1ob05328k日期:——cleavage in hydrazines is widely used in the preparation of amines and thus occupies a significant place in organic synthesis. In this paper, we report a new method for the reductive cleavage of N–N bonds in hydrazines by commercially available and cheap aqueous titanium(III) trichloride. The reaction proceeds smoothly under a broad pH range from acidic to neutral and basic conditions to afford amines in good
-
Enamine chemistry. Part III. Reaction of αβ-unsaturated acid chlorides with enamines of acyclic ketones. Preparation of cyclohexane-1,3-diones作者:J. R. Hargreaves、P. W. Hickmott、B. J. HopkinsDOI:10.1039/j39680002599日期:——isopropyl ketone and the dimethylamine enamine of di-isopropyl ketone gave 2,2,4-trimethyl- and 2,2,4,4-tetramethyl-cyclohexane-1,3-diones respectively. The morpholine enamine of dibenzyl ketone gave 3-morpholino-2,4-diphenyl-cyclohex-2-enone which could not be hydrolysed to the cyclohexane-1,3-dione. The mechanism of the reaction is discussed.
表征谱图
-
氢谱1HNMR
-
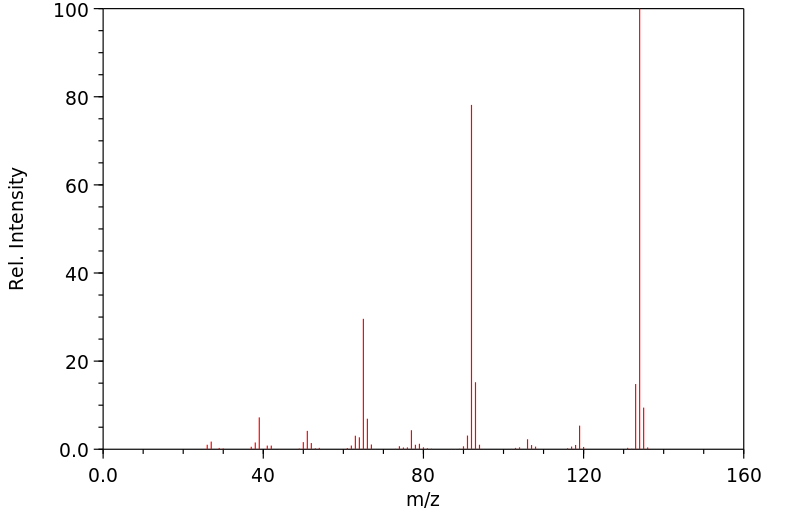
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫