反式-2-己烯酸乙酯 | 27829-72-7
中文名称
反式-2-己烯酸乙酯
中文别名
反-2-己烯酸乙酯;E-2-己烯酸乙脂,反-己-2-烯酸乙酯;E-2-己烯酸乙脂;反式2-己烯酸乙酯
英文名称
ethyl (E)-hex-2-enoate
英文别名
ethyl (E)-2-hexenoate;ethyl trans-2-hexenoate;ethyl (2E)-hexenoate;(E)-ethyl 2-hexenoate
CAS
27829-72-7
化学式
C8H14O2
mdl
——
分子量
142.198
InChiKey
SJRXWMQZUAOMRJ-VOTSOKGWSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:−2 °C(lit.)
-
沸点:123-126 °C12 mm Hg(lit.)
-
密度:0.95 g/mL at 25 °C(lit.)
-
闪点:>230 °F
-
LogP:2.91
-
物理描述:Clear colourless liquid; fruity aroma
-
溶解度:Practically insoluble to insoluble in water
-
折光率:1.430-1.440
-
保留指数:1020;1018;1023;1020;1024;1025;1026;1019;1024
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解。请避免接触氧化物、酸、碱以及还原剂。
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:10
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:0.62
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
TSCA:Yes
-
危险等级:3
-
危险品标志:C
-
安全说明:S26,S27,S28,S36/37/39,S36/39,S45
-
危险类别码:R34
-
WGK Germany:2
-
海关编码:29171900
-
危险品运输编号:UN 3265 8/PG 2
-
RTECS号:MP7750000
-
包装等级:III
-
危险类别:3
-
危险性防范说明:P210,P280,P370+P378,P403+P235,P501
-
危险性描述:H225
-
储存条件:请确保贮藏器密封,并将其存放在阴凉、干燥处,置于一个紧密封装的容器中。
SDS
反-2-己烯酸乙酯 修改号码:5
模块 1. 化学品
产品名称: Ethyl trans-2-Hexenoate
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第4级
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 警告
危险描述 可燃液体
防范说明
[预防] 远离明火/热表面。
穿戴防护手套/护目镜/防护面具。
[储存] 存放于通风良好处。保持凉爽。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 反-2-己烯酸乙酯
百分比: >97.0%(GC)
CAS编码: 27829-72-7
俗名: trans-2-Hexenoic Acid Ethyl Ester
分子式: C8H14O2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
反-2-己烯酸乙酯 修改号码:5
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离明火和热表面。
采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 使用封闭系统,通风。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 液体
外观: 透明
颜色: 无色-几乎无色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 85 °C/3.3kPa
闪点: 62°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.90
反-2-己烯酸乙酯 修改号码:5
模块 9. 理化特性
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 明火
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
反-2-己烯酸乙酯 修改号码:5
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: Ethyl trans-2-Hexenoate
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第4级
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 警告
危险描述 可燃液体
防范说明
[预防] 远离明火/热表面。
穿戴防护手套/护目镜/防护面具。
[储存] 存放于通风良好处。保持凉爽。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 反-2-己烯酸乙酯
百分比: >97.0%(GC)
CAS编码: 27829-72-7
俗名: trans-2-Hexenoic Acid Ethyl Ester
分子式: C8H14O2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
反-2-己烯酸乙酯 修改号码:5
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离明火和热表面。
采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 使用封闭系统,通风。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 液体
外观: 透明
颜色: 无色-几乎无色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 85 °C/3.3kPa
闪点: 62°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.90
反-2-己烯酸乙酯 修改号码:5
模块 9. 理化特性
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 明火
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
反-2-己烯酸乙酯 修改号码:5
模块16 - 其他信息
N/A
制备方法与用途
食品添加剂最大允许使用量及最大允许残留量标准
添加剂信息
- 添加剂中文名称: 反式-2-己烯酸乙酯
- 允许使用该种添加剂的食品中文名称: 食品
- 添加剂功能: 食品用香料
- 最大允许使用量(g/kg): 用于配制香精的各香料成分不得超过在GB 2760中的最大允许使用量和最大允许残留量
此标准确保了食品添加剂的安全与合规使用。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— ethyl (E)-6-iodo-2-hexenoate 64277-92-5 C8H13IO2 268.095 反式-2-己烯酸 2-hexenoic acid 1191-04-4 C6H10O2 114.144 反式-2-己烯酸 (E)-2-Hexenoic acid 13419-69-7 C6H10O2 114.144 —— ethyl (Z)-2-bromo-2-hexenoate 102575-02-0 C8H13BrO2 221.094 山梨酸乙酯 (2E,4E)-ethyl hexa-2,4-dienoate 2396-84-1 C8H12O2 140.182 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (+/-)-4-bromo-hex-2t-enoic acid ethyl ester 53662-68-3 C8H13BrO2 221.094 乙酸反-2-己烯酯 (E)-2-hexen-1-yl acetate 2497-18-9 C8H14O2 142.198 —— ethyl (Z)-2-aminohex-2-enoate 71385-55-2 C8H15NO2 157.213 —— ethyl (Z)-2-bromo-2-hexenoate 102575-02-0 C8H13BrO2 221.094 3-己烯酸乙酯 ethyl hex-3-enoate 2396-83-0 C8H14O2 142.198 3-己烯酸乙酯 ethyl (E)-3-hexenoate 26553-46-8 C8H14O2 142.198 (Z)-己-3-烯酸乙酯 ethyl (3Z)-hex-3-enoate 64187-83-3 C8H14O2 142.198 N-丁酸(反-2-己烯基)酯 trans-2-hexenyl butyrate 53398-83-7 C10H18O2 170.252
反应信息
-
作为反应物:描述:参考文献:名称:(E)-α,β-不饱和酯转化为相应的β,γ-不饱和酯中的“合成效应”摘要:研究了在 HMPA 存在下用六甲基二硅叠氮化锂处理将 (E)-α,β-不饱和酯转化为相应的 β,γ-不饱和酯的立体化学。所得β,γ-不饱和酯的Z/E比率根据(E)-α,β-不饱和酯的γ-取代基而变化。这种现象被“合成效应”合理化,这可能主要归因于 σ→π* 相互作用和/或 6π-电子同芳香性。DOI:10.1246/cl.2003.778
-
作为产物:参考文献:名称:胶态钯易于在有机溶剂中形成,是用于选择性加氢和脱卤化氢的高活性和稳定催化剂摘要:由Pd(hfacac)2和硅烷或含Si-H的聚合物在有机溶剂中形成的胶体Pd是用于氢化和脱卤化氢的活性,稳定和选择性的催化剂。DOI:10.1016/s0040-4039(00)93383-5
文献信息
-
Selective hydrogenation of α,β-unsaturated carbonyl compounds on silica-supported copper nanoparticles作者:Jorge Mendes-Burak、Behnaz Ghaffari、Christophe CopéretDOI:10.1039/c8cc08457b日期:——Silica-supported copper nanoparticles prepared via surface organometallic chemistry are highly efficient for the selective hydrogenation of various α,β-unsaturated carbonyl compounds yielding the corresponding saturated esters, ketones, and aldehydes in the absence of additives. High conversions and selectivities (>99%) are obtained for most substrates upon hydrogenation at 100–150 °C and under 25
-
ORGANOCATALYTIC PROCESS FOR ASYMMETRIC SYNTHESIS OF DECANOLIDES申请人:Council of Scientific and Industrial Research公开号:US20150210665A1公开(公告)日:2015-07-30The present invention discloses organocatalytic process for asymmetric synthesis of highly enantioselective decanolide compounds in high yield with >99% ee. Further, the present invention disclose cost effective, improved organocatalytic process for asymmetric synthesis of highly enantioselective decanolides compounds from non-chiral, cheap, easily available raw materials.本发明公开了一种高效的有机催化过程,用于非对称合成高对映选择性的癸内酯化合物,产量高且对映体过量大于99%。此外,本发明还揭示了一种成本效益高、改进的有机催化过程,用于从非手性、便宜且易于获得的原料中非对称合成高对映选择性的癸内酯化合物。
-
A Chiral 6-Membered <i>N</i>-Heterocyclic Carbene Copper(I) Complex That Induces High Stereoselectivity作者:Jin Kyoon Park、Hershel H. Lackey、Matthew D. Rexford、Kirill Kovnir、Michael Shatruk、D. Tyler McQuadeDOI:10.1021/ol1021756日期:2010.11.5A chiral 6-membered annulated N-heterocyclic (6-NHC) copper complex that catalyzes β-borylations with high yield and enantioselectivity was developed. The chiral 6-NHC copper complex is easy to prepare on the gram scale and is very active, showing 10 000 turnovers at 0.01 mol % of catalyst without significant decrease of enantioselectivity and with useful reaction rates.
-
A simple synthetic route to substituted cyclopentenolones作者:Richard T. Brown、Walter P. Blackstock、Mark WingfieldDOI:10.1016/s0040-4039(01)90053-x日期:——Preparation of novel cyclopent-3-ene-1,2-dione dimers from γ-substituted crotonate esters and dimethyl oxalate by vinylogous double Claisen condensations has given access to a series of polyfunctional cyclopentane derivatives potentially useful in synthesis.
-
Borane-Catalyzed Reductive α-Silylation of Conjugated Esters and Amides Leaving Carbonyl Groups Intact作者:Youngchan Kim、Sukbok ChangDOI:10.1002/anie.201508669日期:2016.1.4valuable α‐silyl carbonyl products. The α‐silylation occurs chemoselectively, thus leaving the labile carbonyl groups intact. The reaction features a broad scope of both acyclic and cyclic substrates, and the synthetic utility of the obtained α‐silyl carbonyl products is also demonstrated. Mechanistic studies revealed two operative steps: fast 1,4‐hydrosilylation of conjugated carbonyls and then slow
表征谱图
-
氢谱1HNMR
-
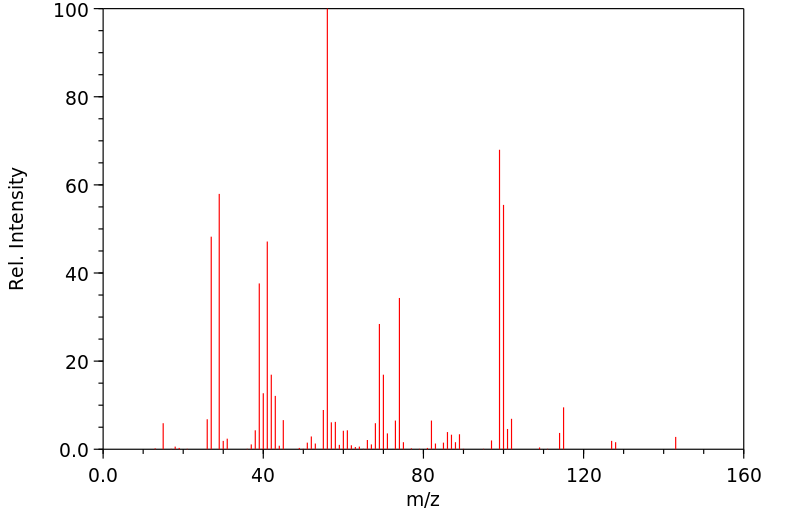
质谱MS
-
碳谱13CNMR
-
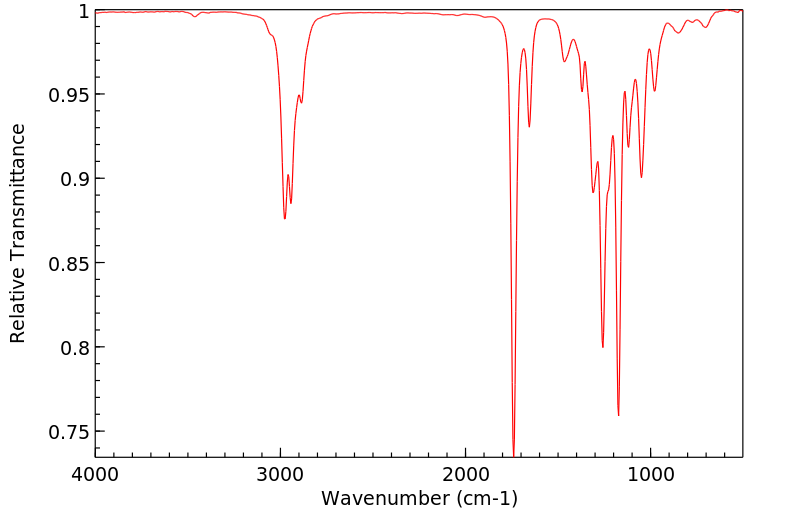
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯