喹啉-4-硼酸 | 371764-64-6
中文名称
喹啉-4-硼酸
中文别名
4-喹啉硼酸;喹啉-4-硼酸盐酸盐
英文名称
quinolin-4-ylboronic acid
英文别名
quinoline-4-boronic acid;4-quinolineboronic acid;4-quinolinylboronic acid;4-quinolylboronic acid
CAS
371764-64-6
化学式
C9H8BNO2
mdl
MFCD03095174
分子量
172.979
InChiKey
KATIRQRAVXTBNY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:400.3±37.0 °C(Predicted)
-
密度:1.28
计算性质
-
辛醇/水分配系数(LogP):1.73
-
重原子数:13
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:53.4
-
氢给体数:2
-
氢受体数:3
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36/37/39
-
危险类别码:R36/37/38
-
海关编码:2933499090
-
危险性防范说明:P261,P280,P305+P351+P338
-
危险性描述:H302,H315,H319,H332,H335
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: Quinoline-4-boronic acid
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H315: Causes skin irritation
H318: Causes serious eye damage
H335: May cause respiratory irritation
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
P280: Wear protective gloves/protective clothing/eye protection/face protection
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
Section 3. Composition/information on ingredients.
Ingredient name: Quinoline-4-boronic acid
CAS number: 371764-64-6
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, under −20◦C.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
No data
Boiling point:
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C9H8BNO2
Molecular weight: 173.0
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: Quinoline-4-boronic acid
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H315: Causes skin irritation
H318: Causes serious eye damage
H335: May cause respiratory irritation
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
P280: Wear protective gloves/protective clothing/eye protection/face protection
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
Section 3. Composition/information on ingredients.
Ingredient name: Quinoline-4-boronic acid
CAS number: 371764-64-6
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, under −20◦C.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
No data
Boiling point:
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C9H8BNO2
Molecular weight: 173.0
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:喹啉-4-硼酸 在 四(三苯基膦)钯 、 sodium carbonate 、 三氟乙酸 作用下, 以 乙二醇二甲醚 、 二氯甲烷 、 水 、 N,N-二甲基甲酰胺 、 乙腈 为溶剂, 反应 28.0h, 生成 5-(4-(piperazin-1-yl)phenyl)-3-(quinolin-4-yl)pyridin-2-amine参考文献:名称:Structure–Activity Relationship of 3,5-Diaryl-2-aminopyridine ALK2 Inhibitors Reveals Unaltered Binding Affinity for Fibrodysplasia Ossificans Progressiva Causing Mutants摘要:There are currently no effective therapies for fibrodysplasia ossificans progressiva (FOP), a debilitating and progressive heterotopic ossification disease caused by activating mutations of ACVR1 encoding the BMP type I receptor kinase ALK2. Recently, a subset of these same mutations of ACVR1 have been identified in-diffuse intrinsic pontine ghoma (DIPG) tumors. Here we describe the structure-activity relationship for a series of novel ALK2 inhibitors based on the 2-aminopyridine compound K02288. Several modifications increased potency in kinase, thermal shift, or cell-based assays of BMP signaling and transcription, as well as selectivity for ALK2 versus closely related BMP AND TGF-beta type I receptor kinases. Compounds in this series exhibited a wide range of in vitro cytotoxicity that was not correlated with potency or selectivity, suggesting mechanisms independent of BMP or TGF-beta inhibiton. This study also highlights a potent 2-methylpyridine derivative 10 (LDN 214117) with a high degree of selectivity for ALK2 and low cytotoxicity that could provide a template for preclinical development. contrary to the notion that activating mutations of ALK2 might alter inhibitor efficacy due to potential conformational changes in the ATP-binding site, the compounds demonstrated consistent binding to a panel of mutant and wild-type ALK2 proteins. Thus, BMP inhibitors identified via activity against wild-type ALK2 signaling are likely to be of clinical relevance for the diverse ALK2 mutant proteins associated with FOP and DIPG.DOI:10.1021/jm501177w
-
作为产物:描述:4-溴喹啉 在 四羟基二硼 、 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) 、 N,N-二异丙基乙胺 、 三苯基膦 作用下, 以 乙醇 为溶剂, 反应 4.0h, 生成 喹啉-4-硼酸参考文献:名称:镍催化四羟基二硼 [B2(OH)4] 卤化物和拟卤化物的硼化反应摘要:芳基硼酸作为试剂和目标结构在各种有用的应用中变得越来越重要。最近,报道了使用原子经济的四羟基二硼(BBA)试剂在钯催化下合成芳基硼酸。钯的高成本,加上钯和铜催化工艺的一些限制,促使我们开发一种替代方法。因此,已经制定了使用四羟基二硼(BBA)对芳基和杂芳基卤化物和拟卤化物进行镍催化的硼基化反应。该反应被证明具有广泛的官能团耐受性并适用于许多杂环体系。据我们所知,这里提供的例子代表了在室温下进行的唯一有效的镍催化宫浦硼化反应。DOI:10.1021/jo401104y
文献信息
-
METALLO-BETA-LACTAMASE INHIBITORS申请人:Merck Sharp & Dohme Corp.公开号:US20160333021A1公开(公告)日:2016-11-17The present invention relates to compounds of formula (I) that are metallo-β-lactamase inhibitors, the synthesis of such compounds, and the use of such compounds for use with β-lactam antibiotics for overcoming resistance.本发明涉及式(I)的化合物,这些化合物是金属β-内酰胺酶抑制剂,以及这些化合物的合成和与β-内酰胺类抗生素一起用于克服耐药性的用途。
-
INHIBITORS OF FIBROBLAST ACTIVATION PROTEIN申请人:Praxis Biotech LLC公开号:US20190185451A1公开(公告)日:2019-06-20Compounds and compositions for modulating fibroblast activation protein (FAP) are described. The compounds and compositions may find use as therapeutic agents for the treatment of diseases, including hyperproliferative diseases.描述了用于调节成纤维细胞活化蛋白(FAP)的化合物和组合物。这些化合物和组合物可能被用作治疗疾病的治疗剂,包括高增殖性疾病。
-
[EN] cGAS ANTAGONIST COMPOUNDS<br/>[FR] COMPOSÉS ANTAGONISTES DU CGAS申请人:IMMUNE SENSOR LLC公开号:WO2017176812A1公开(公告)日:2017-10-12Disclosed are novel compounds of Formula (I) that are cGAS antagonists, methods of preparation of the compounds, pharmaceutical compositions comprising the compounds, and their use in medical therapy.揭示了一种新型化合物的化学式(I),这些化合物是cGAS拮抗剂,涉及到这些化合物的制备方法、包含这些化合物的药物组合物,以及它们在医学治疗中的应用。
-
[EN] ARYL-BIPYRIDINE AMINE DERIVATIVES AS PHOSPHATIDYLINOSITOL PHOSPHATE KINASE INHIBITORS<br/>[FR] DÉRIVÉS D'AMINE ARYL-BIPYRIDINE UTILISÉS EN TANT QU'INHIBITEURS DE LA PHOSPHATIDYLINOSITOL PHOSPHATE KINASE申请人:PETRA PHARMA CORP公开号:WO2019126733A1公开(公告)日:2019-06-27The invention relates to inhibitors of PI5P4K inhibitors useful in the treatment of cancers, neurodegenerative diseases, inflammatory disorders, and metabolic diseases, having the Formula (I): wherein A, X, Y, Z, Q, R1, R2, R3, R4, R5, and n are described herein.
-
作为URAT1抑制剂的噻吩类衍生物
表征谱图
-
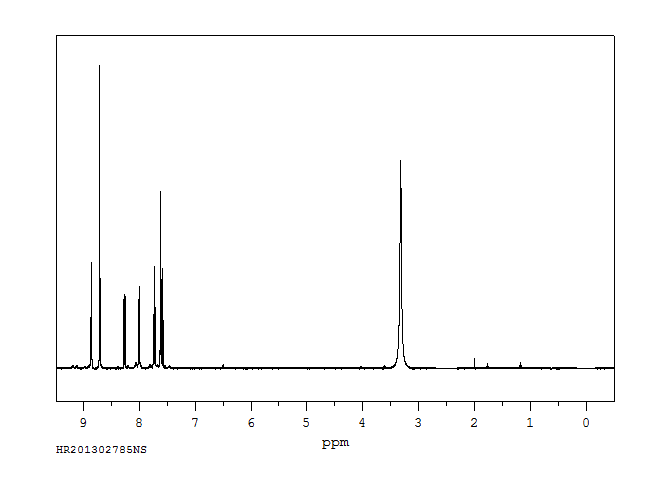
氢谱1HNMR
-
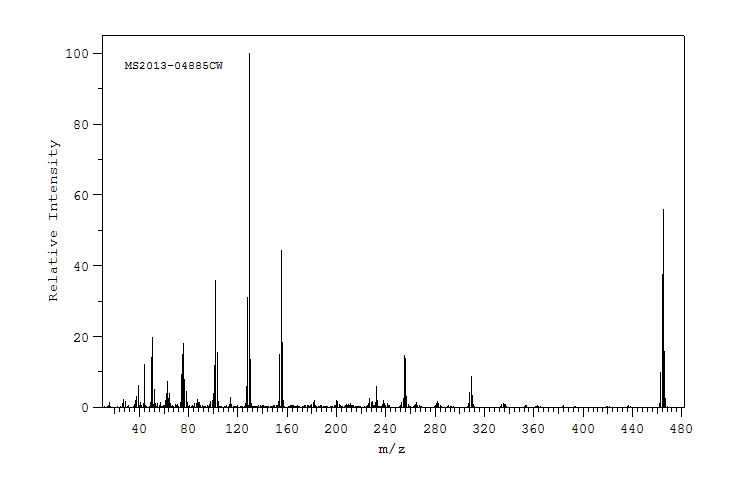
质谱MS
-
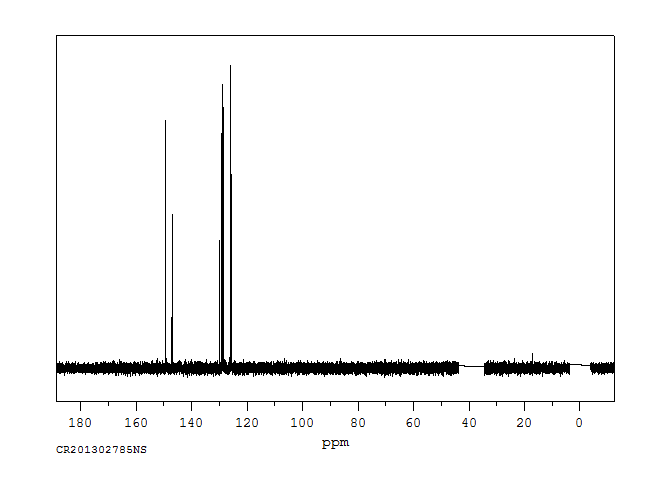
碳谱13CNMR
-
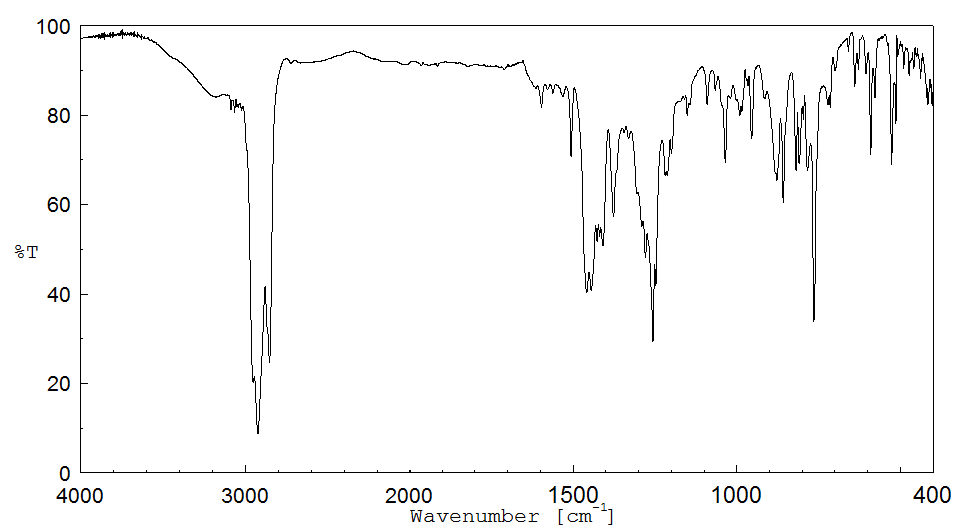
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-(叔丁基)-2-(喹啉-2-基)-4,5-二氢噁唑
(SP-4-1)-二氯双(喹啉)-钯
(E)-2-氰基-3-[5-(2,5-二氯苯基)呋喃-2-基]-N-喹啉-8-基丙-2-烯酰胺
(8α,9S)-(+)-9-氨基-七氢呋喃-6''-醇,值90%
(6,7-二甲氧基-4-(3,4,5-三甲氧基苯基)喹啉)
(1-羟基-5-硝基-8-氧代-8,8-dihydroquinolinium)
黄尿酸 8-甲基醚
麻保沙星EP杂质D
麻保沙星EP杂质B
麻保沙星EP杂质A
麦角腈甲磺酸盐
麦角腈
麦角灵
麦皮星酮
麦特氧特
高铁试剂
高氯酸3-苯基[1,3]噻唑并[3,2-f]5-氮杂菲-4-正离子
马波沙星EP杂质F
马波沙星
马来酸茚达特罗杂质
马来酸茚达特罗
马来酸维吖啶
马来酸来那替尼
马来酸四甲基铵
香草木宁碱
颜料红R-122
颜料红210
颜料红
顺式-苯并(f)喹啉-7,8-二醇-9,10-环氧化物
顺式-(alphaR)-N-(4-氯苯基)-4-(6-氟-4-喹啉基)-alpha-甲基环己烷乙酰胺
非那沙星
非那沙星
青花椒碱
青色素863
雷西莫特
隐花青
阿莫地喹-d10
阿莫地喹
阿莫吡喹N-氧化物
阿美帕利
阿米诺喹
阿立哌唑溴代杂质
阿立哌唑杂质B
阿立哌唑杂质38
阿立哌唑杂质1750
阿立哌唑杂质13
阿立哌唑杂质
阿立哌唑杂质
阿尔马尔
阿加曲班杂质43