(1-戊基)三苯基溴化磷 | 21406-61-1
中文名称
(1-戊基)三苯基溴化磷
中文别名
戊基三苯基溴化磷;正戊基三苯基溴化膦;N-戊基三苯基溴化膦;戊基三苯基鏻溴化物;正戊基三苯基溴化磷
英文名称
pentyltriphenylphosphonium bromide
英文别名
n-pentyltriphenylphosphonium bromide;(1-pentyl)triphenylphosphonium bromide;pentyltriphenylphosphanium bromide;pentyl(triphenyl)phosphanium;bromide
CAS
21406-61-1
化学式
Br*C23H26P
mdl
——
分子量
413.337
InChiKey
VAUKWMSXUKODHR-UHFFFAOYSA-M
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:165-168 °C
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,请避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):2.17
-
重原子数:25
-
可旋转键数:7
-
环数:3.0
-
sp3杂化的碳原子比例:0.22
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险品标志:Xn
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
海关编码:2931900090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:将贮藏器密封后,放入一个紧密封装的容器中,并储存在阴凉、干燥的地方。
SDS
模块 1. 化学品
1.1 产品标识符
: PentyltriphenylphOSphonium bromide
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS分类
皮肤刺激 (类别 2)
眼睛刺激 (类别 2A)
特异性靶器官系统毒性(一次接触) (类别 3)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H315 造成皮肤刺激。
H319 造成严重眼刺激。
H335 可能造成呼吸道刺激。
警告申明
预防措施
P261 避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾。
P264 操作后彻底清洗皮肤。
P271 只能在室外或通风良好之处使用。
P280 戴防护手套/戴防护眼罩/戴防护面具。
事故响应
P302 + P352 如果皮肤接触:用大量肥皂和水清洗。
P304 + P340 如误吸入:将受害人转移到空气新鲜处,保持呼吸舒适的休息姿势。
P305 + P351 + P338 如进入眼睛:用水小心冲洗几分钟。如戴隐形眼镜并可方便地取出,取出
隐形眼镜。继续冲洗。
P312 如感觉不适,呼叫解毒中心或医生。
P321 具体治疗(见本标签上提供的急救指导)。
P332 + P313 如发生皮肤刺激:求医/就诊。
P337 + P313 如仍觉眼刺激:求医/就诊。
P362 脱掉沾污的衣服。
储存
P403 + P233 存放在通风良好的地方。保持容器密闭。
P405 存放处须加锁。
废弃处置
P501 将内装物/容器送到批准的废物处理厂处理。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C23H26P · Br
分子式
: 413.34 g/mol
分子量
组分 浓度或浓度范围
PentyltriphenylphOSphonium bromide
化学文摘登记号(CAS 21406-61-1 <= 100 %
No.) 244-374-5
EC-编号
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 向到现场的医生出示此安全技术说明书。
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
切勿给失去知觉者喂食任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 磷的氧化物, 溴化氢气
5.3 给消防员的建议
如有必要,佩戴自给式呼吸器进行消防作业。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
使用个人防护装备。 避免粉尘生成。 避免吸入蒸气、气雾或气体。 保证充分的通风。
将人员疏散到安全区域。 避免吸入粉尘。
6.2 环境保护措施
不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
收集和处置时不要产生粉尘。 扫掉和铲掉。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 避免形成粉尘和气溶胶。
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 控制参数
职业接触限值
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
按照良好的工业卫生和安全规范进行操作。 休息前及工作结束时洗手。
个体防护装备
眼面防护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国
143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143)
防毒罐。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/熔点范围: 167 - 168 °C
f) 初沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸气密度
无数据资料
m) 密度/相对密度
无数据资料
n) 水溶性
无数据资料
o) 正辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 黏度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤腐蚀/刺激
中度的皮肤刺激 前面的数据或数据判读是根据定量结构活性关系(QSAR)确定的。
严重眼睛损伤/眼刺激
中度的眼睛刺激 前面的数据或数据判读是根据定量结构活性关系(QSAR)确定的。
呼吸或皮肤过敏
无数据资料
生殖细胞致突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
吸入 - 可能造成呼吸道刺激。
前面的数据或数据判读是根据定量结构活性关系(QSAR)确定的。
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危害
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
食入 吞咽可能有害。
皮肤 通过皮肤吸收可能有害。 引起皮肤刺激。
眼睛 造成严重眼刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT和vPvB的结果评价
无数据资料
12.6 其他不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
联系专业的拥有废弃物处理执照的机构来处理此物质。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国运输名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 特殊防范措施
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
反应信息
-
作为反应物:描述:(1-戊基)三苯基溴化磷 在 palladium on activated charcoal 吡啶 、 sodium chlorite 、 sodium dihydrogenphosphate 、 2-甲基-2-丁烯 、 偶氮二甲酸二异丙酯 、 氢气 、 sodium amide 、 苯磺酰氯 、 间氯过氧苯甲酸 、 三苯基膦 作用下, 以 四氢呋喃 、 乙醚 、 叔丁醇 为溶剂, 反应 34.0h, 生成 奥利司他参考文献:名称:恶唑啉N-氧化物介导的[2 + 3]环加成。在合成(-)-四氢脂肪抑制素中的应用。摘要:[反应:见正文]樟脑衍生的恶唑啉N-氧化物与α,β-不饱和酯的[2 + 3]环加成反应得到加合物8。从该化合物按九步反应序列制备四氢lipstatin 1。DOI:10.1021/ol990734k
-
作为产物:参考文献:名称:Ir 催化未活化内烯烃与苯苯胺通过 C-H 活化和连续异构化进行远端支链选择性加氢芳基化摘要:我们在此报告了一种由Ir催化剂催化的C-H活化和连续异构化的协同策略,以选择性地获得支化异构体作为苯甲酰苯胺衍生物的C-H烷基化产物。良好调节的配体和导向基团对于实现这种选择性至关重要。该反应的范围通过使用各种取代基和复杂分子来证明。DOI:10.1021/acs.orglett.3c01619
-
作为试剂:描述:参考文献:名称:Stereochemistry of allylic oxidation with selenium dioxide. Stereospecific oxidation of gem-dimethyl olefins摘要:DOI:10.1021/ja00748a028
文献信息
-
[EN] 2-CYCLOALKYL RESORCINOL CANNABINERGIC LIGANDS<br/>[FR] LIGANDS CANNABINERGIQUES DE 2-CYCLOALKYL RÉSORCINOL申请人:UNIV NORTHEASTERN公开号:WO2014062965A1公开(公告)日:2014-04-24The present invention relates to novel 2-cycloalkyl resorcinol compounds; to pharmaceutical compositions comprising the compounds; and to methods of preparing the compounds and uses thereof. The disclosed compounds can bind to and modulate the cannabinoid receptors and thus, they are specific ligands for these receptors. The invented compounds, when administered in a therapeutically effective amount to an individual or animal, results in a sufficiently high level of that compound in the individual or animal to cause a physiological response. The physiological response may be useful to treat a number of physiological conditions.
-
Synthese von Alkylphenolen und -pyrocatecholen aus<i>Plectranthus albidus</i>(<i>Labiatae</i>)
-
Stereoinversion of Unactivated Alcohols by Tethered Sulfonamides作者:Paul T. Marcyk、Latisha R. Jefferies、Deyaa I. AbuSalim、Maren Pink、Mu‐Hyun Baik、Silas P. CookDOI:10.1002/anie.201812894日期:2019.2.4remains an undeveloped area of organic synthesis. Moreover, catalytic activation of this difficult electrophile with predictable stereo-outcomes presents an even more formidable challenge. Described herein is a simple iron-based catalyst system which provides the mild, direct conversion of secondary and tertiary alcohols to sulfonamides. Starting from enantioenriched alcohols, the intramolecular variant
-
Ionic and Organometallic Reductions with N-Heterocyclic Carbene Boranes作者:Qianli Chu、Malika Makhloufâ Brahmi、Andrey Solovyev、Shau-Hua Ueng、Dennisâ P. Curran、Max Malacria、Louis Fensterbank、Emmanuel LacôteDOI:10.1002/chem.200902450日期:2009.12.7Surgical reduction: N‐Heterocyclic carbene–borane complexes such as depicted are neutral, organic soluble analogues of borohydride anions with a weak hydridic character, compatible with organometallic catalysis. They are applicable for surgical reductions in complex, multifunctional molecules.
-
Process for Preparation of Hexadecyl Cis-9-Tetradecenoate and Hexadecyl Cis-10-Tetradecenoate申请人:Bethala Lakshmi Anu Prabhavathi Devi公开号:US20110269987A1公开(公告)日:2011-11-03Hexadecyl cis-9-tetradecenoate commonly known as Cetyl myristoleate (CMO) is being used for the treatment of osteoarthritis and other joint inflammatory diseases, cis-9-Tetradecenoic acid (cis-9-myristoleic acid) is the main precursor for the preparation of CMO. As there are limited natural plant sources for cis-9-tetradecenoic acid, the present invention aimed at the synthesis of cis-9-tetradecenoic acid methyl ester from oleic acid methyl ester. As oleic acid is not available in pure form, this has to be isolated from oleic acid-rich oils like olive oil. cis-10-Tetradecenoic acid methyl ester, an isomer of cis-9-tetradecenoic acid was also prepared from undecenoic acid methyl ester, a derivative of castor oil. Undecenoic acid is easily available commercially in pure form. Hexadecyl cis-9-tetradecenoate and hexadecyl c/s-10-tetradecenoate were prepared by enzymatic transesterification of cis-9-tetradecenoic acid methyl ester and cis-10-tetradecenoic acid methyl ester with 1-hexadecanol (cetyl alcohol) respectively. Both the isomers of cetyl myristoleate were evaluated for anti arthritis, blocking inflammation and reduction of adjuvant-induced arthritis in rats.十六烷基顺-9-十四碳烯酸盐通常被称为鲸蜡醇豆蔻酸酯(CMO),目前正被用于治疗骨关节炎和其他关节炎症疾病,顺-9-十四碳烯酸(顺-9-豆蔻酸)是制备CMO的主要前体。由于顺-9-十四碳烯酸的自然植物来源有限,本发明旨在从油酸甲酯合成顺-9-十四碳烯酸甲酯。由于油酸无法以纯净形式获得,因此需要从富含油酸的油类如橄榄油中提取。还从十一烯酸甲酯,即蓖麻油的衍生物,制备了顺-10-十四碳烯酸甲酯,这是顺-9-十四碳烯酸的异构体。十一烯酸在市场上容易以纯净形式获得。通过酶促转酯反应,分别用顺-9-十四碳烯酸甲酯和顺-10-十四碳烯酸甲酯与1-十六醇(鲸蜡醇)反应,制备了十六烷基顺-9-十四碳烯酸盐和十六烷基顺-10-十四碳烯酸盐。这两种鲸蜡醇豆蔻酸酯的异构体都进行了抗关节炎、阻断炎症和在大鼠中减少佐剂诱导关节炎的评价。
表征谱图
-
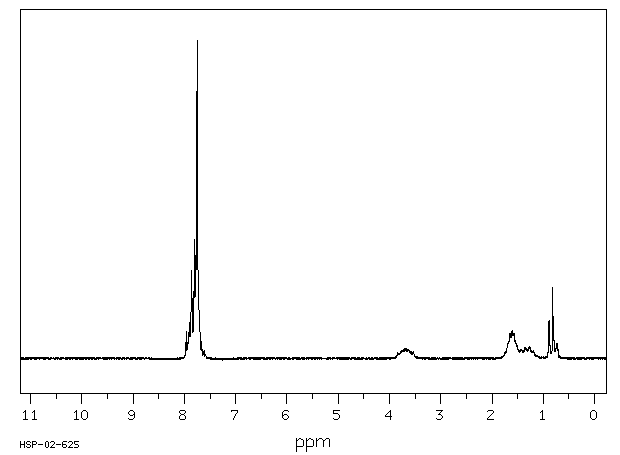
氢谱1HNMR
-
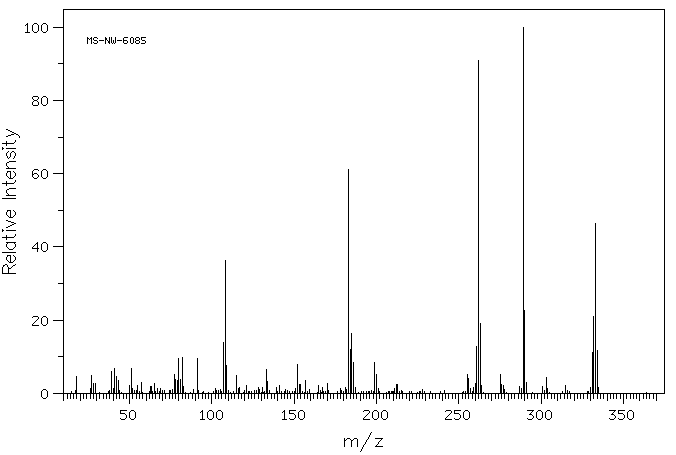
质谱MS
-
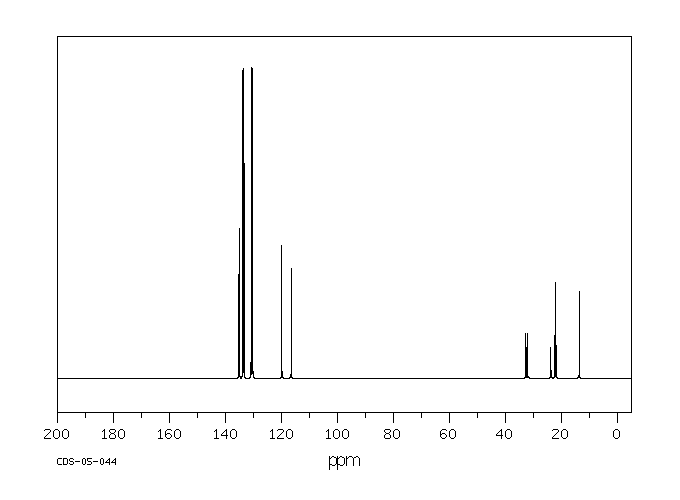
碳谱13CNMR
-
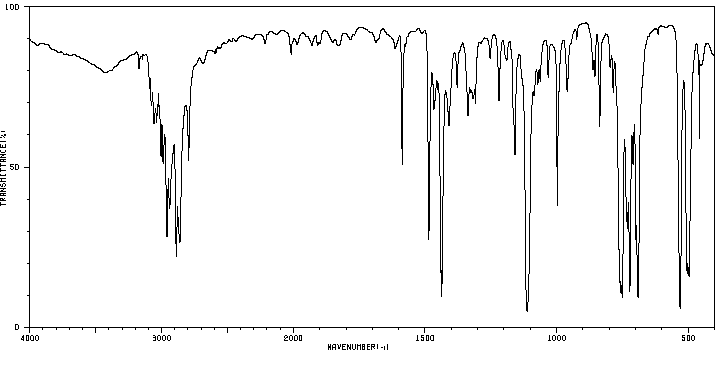
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫