己基β-D-吡喃葡萄糖苷 | 59080-45-4
中文名称
己基β-D-吡喃葡萄糖苷
中文别名
己基BETA-D-吡喃葡萄糖苷;己基Β-D-吡喃葡萄糖苷;己基-Β-D-吡喃葡糖苷;己基Beta-D-吡喃葡萄糖苷
英文名称
n-hexyl-β-D-glucopyranoside
英文别名
hexyl β-D-glucopyranoside;hexyl-1-O-β-glucopyranoside;n-hexyl beta-D-glucopyranoside;Hexyl beta-D-glucopyranoside;(2R,3R,4S,5S,6R)-2-hexoxy-6-(hydroxymethyl)oxane-3,4,5-triol
CAS
59080-45-4
化学式
C12H24O6
mdl
——
分子量
264.319
InChiKey
JVAZJLFFSJARQM-RMPHRYRLSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:84-86°C
-
沸点:432.3±45.0 °C(Predicted)
-
密度:1.23±0.1 g/cm3(Predicted)
-
溶解度:可溶于丙酮、乙醇、水
计算性质
-
辛醇/水分配系数(LogP):0.1
-
重原子数:18
-
可旋转键数:7
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:99.4
-
氢给体数:4
-
氢受体数:6
安全信息
-
危险品标志:Xi
-
安全说明:S22,S24/25
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:29389090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 戊基BETA-D-吡喃葡萄糖苷 pentyl β-D-glucoside 66957-71-9 C11H22O6 250.292 丁基葡糖苷 n-butyl β-D-glucopyranoside 5391-18-4 C10H20O6 236.265 丙基 BETA-D-吡喃葡萄糖苷 Propyl-β-D-glucopyranosid 34384-77-5 C9H18O6 222.238 庚基-β-D-吡喃葡萄糖苷 heptyl β-D-glucopyranoside 78617-12-6 C13H26O6 278.346 壬基-Β-D-葡萄吡喃糖甙 (2R,3S,4S,5R,6R)-2-Hydroxymethyl-6-nonyloxy-tetrahydro-pyran-3,4,5-triol 69984-73-2 C15H30O6 306.4 n-辛基-β-D-吡喃葡萄糖苷 n-octyl β-D-glucopyranoside 29836-26-8 C14H28O6 292.373 (2R,3R,4S,5R,6R)-2-癸氧基-6-(羟基甲基)四氢吡喃-3,4,5-三醇 n-decyl β-D-glucopyranoside 58846-77-8 C16H32O6 320.426 纤维素二糖 Cellobiose 16462-44-5 C12H22O11 342.3 D-葡萄糖 D-glu 2280-44-6 C6H12O6 180.158 可得然胶 β-D-glucose 492-61-5 C6H12O6 180.158 —— hexan-1-ol 2,3,4,6-tetra-O-acetyl-β-D-glucopyranoside 76870-85-4 C20H32O10 432.468 蔗糖 Sucrose 57-50-1 C12H22O11 342.3 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 戊基BETA-D-吡喃葡萄糖苷 pentyl β-D-glucoside 66957-71-9 C11H22O6 250.292 —— hexane-1,5-diol 1-O-β-D-glucopyranoside —— C12H24O7 280.318 丁基葡糖苷 n-butyl β-D-glucopyranoside 5391-18-4 C10H20O6 236.265 丙基 BETA-D-吡喃葡萄糖苷 Propyl-β-D-glucopyranosid 34384-77-5 C9H18O6 222.238 庚基-β-D-吡喃葡萄糖苷 heptyl β-D-glucopyranoside 78617-12-6 C13H26O6 278.346 壬基-Β-D-葡萄吡喃糖甙 (2R,3S,4S,5R,6R)-2-Hydroxymethyl-6-nonyloxy-tetrahydro-pyran-3,4,5-triol 69984-73-2 C15H30O6 306.4 n-辛基-β-D-吡喃葡萄糖苷 n-octyl β-D-glucopyranoside 29836-26-8 C14H28O6 292.373 (2R,3R,4S,5R,6R)-2-癸氧基-6-(羟基甲基)四氢吡喃-3,4,5-三醇 n-decyl β-D-glucopyranoside 58846-77-8 C16H32O6 320.426 —— hexyl-β–gentiobioside 129277-36-7 C18H34O11 426.461 —— n-Hexyl O-β-D-xylopyranosyl-(1->6)-β-D-glucopyranoside 133084-29-4 C17H32O10 396.435 D-葡萄糖 D-glu 2280-44-6 C6H12O6 180.158 甘露糖 D-Mannose 530-26-7 C6H12O6 180.158 可得然胶 β-D-glucose 492-61-5 C6H12O6 180.158 —— hexan-1-ol 2,3,4,6-tetra-O-acetyl-β-D-glucopyranoside 76870-85-4 C20H32O10 432.468 —— fructopyranose 6347-01-9 C6H12O6 180.158 —— (2R,3R,4S,5S,6R)-2-hexoxy-6-(trityloxymethyl)oxane-3,4,5-triol 808137-22-6 C31H38O6 506.639 —— [(2R,3R,4S,5R,6R)-4,5-dibenzoyloxy-6-hexoxy-2-(hydroxymethyl)oxan-3-yl] benzoate 808137-26-0 C33H36O9 576.643 —— 2-keto-D-glucose —— C6H10O6 178.142 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:Significantly Improved Equilibrium Yield of Long-Chain Alkyl Glucosides via Reverse Hydrolysis in a Water-Poor System Using Cross-Linked Almond Meal as a Cheap and Robust Biocatalyst摘要:An array of ten beta-D-glueopyranosides with varied alkyl chain lengths were enzymatically synthesized. It was found that for longer alkyl chains a lower initial rate and final yield of glucoside was obtained except for methyl glucoside because of the severe toxicity of methanol to the enzyme. From a thermodynamics point of view, the equilibrium constant and Gibbs free energy variation of the glucoside syntheses were systematically investigated. To improve the final yields of the glucosides containing long alkyl chains the equilibrium of the enzymatic glucoside synthesis was altered. The equilibrium yield of decyl beta-D-glucoside increased from 1.9% to 6.1% when the water content was reduced from 10% to 5% (v/v) using tert-butanol as a cosolvent and 0.10 mol/L of glucose as a substrate. As for the other longer alkyl chain glucosides, heptyl beta-D-glucoside was found to have significant surface activity as well.DOI:10.1016/s1872-2067(11)60333-1
-
作为产物:描述:参考文献:名称:Significantly Improved Equilibrium Yield of Long-Chain Alkyl Glucosides via Reverse Hydrolysis in a Water-Poor System Using Cross-Linked Almond Meal as a Cheap and Robust Biocatalyst摘要:An array of ten beta-D-glueopyranosides with varied alkyl chain lengths were enzymatically synthesized. It was found that for longer alkyl chains a lower initial rate and final yield of glucoside was obtained except for methyl glucoside because of the severe toxicity of methanol to the enzyme. From a thermodynamics point of view, the equilibrium constant and Gibbs free energy variation of the glucoside syntheses were systematically investigated. To improve the final yields of the glucosides containing long alkyl chains the equilibrium of the enzymatic glucoside synthesis was altered. The equilibrium yield of decyl beta-D-glucoside increased from 1.9% to 6.1% when the water content was reduced from 10% to 5% (v/v) using tert-butanol as a cosolvent and 0.10 mol/L of glucose as a substrate. As for the other longer alkyl chain glucosides, heptyl beta-D-glucoside was found to have significant surface activity as well.DOI:10.1016/s1872-2067(11)60333-1
文献信息
-
Site-Selective and Product Chemoselective Aliphatic C–H Bond Hydroxylation of Polyhydroxylated Substrates作者:Margarida Borrell、Sergio Gil-Caballero、Massimo Bietti、Miquel CostasDOI:10.1021/acscatal.9b05423日期:2020.4.17Site-selective and product chemoselective aliphatic C–H bond oxidation of 1,2-diols and of polyhydroxylated substrates using iron and manganese catalysts and hydrogen peroxide as terminal oxidant is described. The reaction capitalizes on the use of fluorinated alcohol solvents such as 2,2,2-trifluoroethanol (TFE) and 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP), which exert a strong polarity reversal in介绍了使用铁和锰催化剂以及过氧化氢作为末端氧化剂对1,2-二醇和多羟基化底物进行位点选择性和产物化学选择性的脂肪族CH键的氧化反应。该反应利用了氟化醇溶剂,例如2,2,2-三氟乙醇(TFE)和1,1,1,3,3,3,3-六氟-2-丙醇(HFIP),可产生强烈的极性反转1,2-二醇的羟基部分通过氢键结合,进而转化为针对HAT的近端C-H键的强烈失活,而HAT则通过假定的高价和亲电金属-氧代物质氧化。结果,描述了复杂的多官能分子(例如类固醇,糖和药物)的位点选择性氧化和产物化学选择性氧化,在偏远且未激活的位置发生独家或主要的C–H键羟化反应。本报告公开了在氟化醇溶剂中由HAT引发的羟基化反应,作为显示出对当代醇氧化具有正交化学选择性的方法,为在密集官能化分子中进行合成规划提供了有用的工具。
-
[EN] CONDENSED HETEROCYCLIC COMPOUNDS HAVING 5-HT6 RECEPTOR AFFINITY<br/>[FR] COMPOSÉS HÉTÉROCYCLIQUES CONDENSÉS AYANT UNE AFFINITÉ POUR LE RÉCEPTEUR 5-HT6申请人:MEMORY PHARM CORP公开号:WO2010021797A1公开(公告)日:2010-02-25The present disclosure provides compounds having affinity for the 5-HT6 receptor which are of the formula (I) wherein R1, A, B, D, E, G, Q, Ar, n, m, and p are as defined herein. The disclosure also relates to methods of preparing such compounds, compositions containing such compounds, and methods of use thereof.本公开提供了具有亲和力的化合物,其对5-HT6受体具有亲和力,其化学式为(I),其中R1、A、B、D、E、G、Q、Ar、n、m和p如本文所定义。该公开还涉及制备这种化合物的方法、含有这种化合物的组合物以及使用方法。
-
[EN] 4'-AMINO CYCLIC COMPOUNDS HAVING 5-HT6 RECEPTOR AFFINITY<br/>[FR] COMPOSÉS 4'-AMINO CYCLIQUES PRÉSENTANT UNE AFFINITÉ POUR LE RÉCEPTEUR 5-HT6申请人:MEMORY PHARM CORP公开号:WO2010024980A1公开(公告)日:2010-03-04The present disclosure provides compounds having affinity for the 5-HT6 receptor which are of the formula (I): formula (I) wherein Cy is selected from formula (Il) and wherein R1, R2, R3, Q, G, Ar, m, n and p are as defined herein. The disclosure also relates to methods of preparing such compounds, compositions containing such compounds, and methods of use thereof.
-
PROCESS FOR STRAIGHTENING KERATIN FIBRES WITH A HEATING MEANS AND DENATURING AGENTS申请人:Philippe Michel公开号:US20100028280A1公开(公告)日:2010-02-04The invention relates to a process for straightening keratin fibres, comprising: (i) a step in which a straightening composition containing at least two denaturing agents is applied to the keratin fibres, (ii) a step in which the temperature of the keratin fibres is raised, using a heating means, to a temperature of between 110 and 250° C.该发明涉及一种直发角蛋白纤维的拉直过程,包括:(i)将至少两种变性剂含有的拉直组合物涂抹到角蛋白纤维上的步骤,(ii)使用加热装置将角蛋白纤维的温度升高至110至250°C的步骤。
-
Chemoenzymatic Synthesis of n-Hexyl and O-.BETA.-D-Xylopyranosyl-(1.RAR.6)-.BETA.-D-glucopyranosides作者:Masashi Kishida、Miho Nishiuchi、Keisuke Kato、Hiroyuki AkitaDOI:10.1248/cpb.52.1105日期:——Direct β-glucosidation between 1,6-octanediol (5) and D-glucose (3) using the immobilized β-glucosidase (EC 3.2.1.21) from almonds with the synthetic prepolymer ENTP-4000 gave a mono-β-glucoside (6) in 61.4% yield, which was converted into the n-hexyl β-D-glucopyranoside (1) by means of a chemoenzymatic method. The coupling of the n-hexyl β-D-glucopyranoside congener (13) and 2,3,4-tri-O-acetyl-β-D-xylosyl congener (14), followed by deprotection, afforded the synthetic n-hexyl O-β-D-xylopyranosyl-(1→6)-β-D-glucopyranoside (2), which was identical to the natural 2 with respect to the spectral data and specific rotation.
表征谱图
-
氢谱1HNMR
-
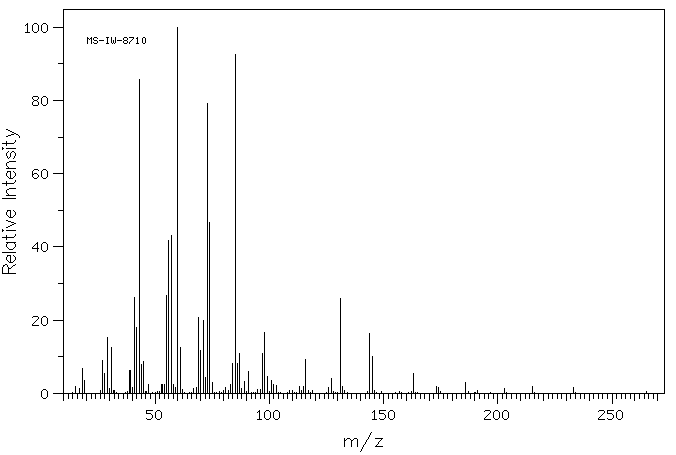
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯