1,2-二甲基咪唑 | 1739-84-0
中文名称
1,2-二甲基咪唑
中文别名
1,2-二甲基-1H-咪唑;1,2-二甲咪唑;N,2-二甲基咪唑
英文名称
1,2-dimethyl-1H-imidazole
英文别名
1,2-dimethylimidazole
CAS
1739-84-0
化学式
C5H8N2
mdl
MFCD00005294
分子量
96.1319
InChiKey
GIWQSPITLQVMSG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:37-39 °C (lit.)
-
沸点:204 °C (lit.)
-
密度:1.084 g/mL at 25 °C (lit.)
-
闪点:198 °F
-
LogP:0.11 at 25℃
-
物理描述:Liquid; OtherSolid
-
保留指数:1006;1006
-
稳定性/保质期:
在常温常压下保持稳定,应避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):0.4
-
重原子数:7
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:17.8
-
氢给体数:0
-
氢受体数:1
安全信息
-
TSCA:Yes
-
危险等级:8
-
安全说明:S2,S24,S26
-
危险品运输编号:UN 3263 8/PG 2
-
WGK Germany:2
-
海关编码:29332990
-
危险类别:8
-
危险品标志:Xi
-
危险类别码:R22,R41,R38
-
RTECS号:NI4838854
-
包装等级:III
-
危险性防范说明:P501,P260,P270,P264,P280,P303+P361+P353,P301+P330+P331,P363,P301+P312+P330,P304+P340+P310,P305+P351+P338+P310,P405
-
危险性描述:H302,H314
-
储存条件:请将容器密封保存,并存放在阴凉、干燥的地方。
SDS
| Name: | 1 2-Dimethylimidazole 98% Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 1739-84-0 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1739-84-0 | 1,2-Dimethylimidazole | 98 | 217-101-2 |
Risk Phrases: 34
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Causes burns.
Potential Health Effects
Eye:
Causes eye burns.
Skin:
Causes skin burns.
Ingestion:
May cause severe and permanent damage to the digestive tract. Causes gastrointestinal tract burns.
Inhalation:
Causes chemical burns to the respiratory tract.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Get medical aid immediately.
Skin:
In case of contact, immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Get medical aid immediately. Wash clothing before reuse.
Ingestion:
If swallowed, do NOT induce vomiting. Get medical aid immediately.
If victim is fully conscious, give a cupful of water. Never give anything by mouth to an unconscious person.
Inhalation:
If inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Combustible liquid and vapor.
Extinguishing Media:
In case of fire, use water, dry chemical, chemical foam, or alcohol-resistant foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up, then place into a suitable container for disposal. Avoid generating dusty conditions. Remove all sources of ignition. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Do not get in eyes, on skin, or on clothing. Keep container tightly closed. Discard contaminated shoes.
Do not breathe dust. Keep away from heat and flame.
Storage:
Keep container closed when not in use. Store in a cool, dry, well-ventilated area away from incompatible substances. Corrosives area.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1739-84-0: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Chunks
Color: clear to white
Odor: None reported.
pH: Not available.
Vapor Pressure: 1 mmHg @ 20 C
Viscosity: Not available.
Boiling Point: 204 deg C
Freezing/Melting Point: 29 - 30 deg C
Autoignition Temperature: 480 deg C ( 896.00 deg F)
Flash Point: 92 deg C ( 197.60 deg F)
Explosion Limits, lower: N/A
Explosion Limits, upper: N/A
Decomposition Temperature:
Solubility in water: soluble in organic solvents
Specific Gravity/Density: 1.080 g/cm3
Molecular Formula: C5H8N2
Molecular Weight: 96.13
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Ignition sources, dust generation.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1739-84-0: NI4838854 LD50/LC50:
Not available.
Carcinogenicity:
1,2-Dimethylimidazole - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: CORROSIVE SOLID, N.O.S.*
Hazard Class: 8
UN Number: 1759
Packing Group: II
IMO
Shipping Name: CORROSIVE SOLID, N.O.S.
Hazard Class: 8
UN Number: 1759
Packing Group: II
RID/ADR
Shipping Name: CORROSIVE SOLID, N.O.S.
Hazard Class: 8
UN Number: 1759
Packing group: II
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: C
Risk Phrases:
R 34 Causes burns.
Safety Phrases:
S 24 Avoid contact with skin.
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
WGK (Water Danger/Protection)
CAS# 1739-84-0: No information available.
Canada
CAS# 1739-84-0 is listed on Canada's DSL List.
CAS# 1739-84-0 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1739-84-0 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
合成方法
向500mL三口烧瓶中加入128g 2-甲基咪唑和100mL乙二醇单甲醚。油浴加热至120℃,在该温度下滴加320g碳酸二甲酯。控制反应温度在120~125℃范围内,并确保滴加时间维持7~8小时。滴加完毕后,在130℃条件下保温3小时。保温结束后,通过气相色谱分析确认2-甲基咪唑的转化率为76%。
随后,在4.0KPa、60℃条件下进行减压蒸馏,以蒸发除去溶剂。使用精馏装置,在4.0KPa真空度下收集100~110℃范围内的馏分,即可得到高纯度的1,2-二甲基咪唑成品,其收率为72%,经气相色谱分析含量达到99.0%。
化学性质1,2-二甲基咪唑为白色棱状结晶,熔点为29-30℃,密度为1.0800,沸点为204℃。
用途1,2-二甲基咪唑主要用作环氧树脂和其它树脂的固化剂、医药中间体。此外,它还可用作研究用缓冲液、固化剂及有机合成中的原料。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-甲基-2-氯甲基咪唑 2-chloromethyl-1-methylimidazole 19225-92-4 C5H7ClN2 130.577 1-甲基-2-羟甲基-1H-咪唑 (1-methylimidazol-2-yl)methanol 17334-08-6 C5H8N2O 112.131 —— 4-chloro-1,2-dimethyl-1H-imidazole 861362-00-7 C5H7ClN2 130.577 5-溴-1,2-二甲基-1H-咪唑 5-bromo-1,2-dimethyl-1H-imidazole 24134-09-6 C5H7BrN2 175.028 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1-methyl-2-methylthiomethylimidazole 86051-78-7 C6H10N2S 142.225 2-(1-甲基-1H-咪唑基-2-基)乙醇 2-(1-methyl-1H-imidazol-2-yl)ethanol 18994-70-2 C6H10N2O 126.158 5-溴-1,2-二甲基-1H-咪唑 5-bromo-1,2-dimethyl-1H-imidazole 24134-09-6 C5H7BrN2 175.028
反应信息
-
作为反应物:描述:参考文献:名称:同时合成两种离子液体的通用方法,否则难以获得,具有高原子经济性。摘要:提出了一种新的合成方法以及具有烯丙基或乙基取代基以及三氟甲磺酸根,甲苯磺酸根,硫酸甲酯或甲磺酸根阴离子的氮基离子液体的全光谱(NMR,IR,MS)和离子色谱表征(IC)。在16种新型离子液体的样品上,已证明了阴离子交换方法的多功能性。在已经进行的复分解反应中,使用烷基化剂将卤化物阴离子在离子液体中与基于烷基磺酸盐的阴离子交换。讨论了使用离子色谱分析新合成的化合物获得的结果。另外,还提出了利用在易位反应中获得的气态卤代甲烷副产物的利用方法,否则该副产物难以合成。这种方法确保了整个过程的高原子经济性,DOI:10.1002/open.201900217
-
作为产物:参考文献:名称:Balaban; Pyman, Journal of the Chemical Society, 1924, vol. 125, p. 1569摘要:DOI:
-
作为试剂:参考文献:名称:Inhibition of serine proteases: activity of 1,3-diazetidine-2,4-diones摘要:The present work demonstrates that the 1,3-diazetidifie-2,4-dione nucleus is effective as a scaffold of serine protease inhibitors. Compound 1 displayed high activity against human cathepsin G and alpha -chymotrypsin (0.39, 0.69 nM). Compound 6 exhibited 0.85 nM inhibition of human chymase. Compound 10 was a selective inhibitor against human neutrophil elastase. (C) 2001 Elsevier Science Ltd. All rights reserved.DOI:10.1016/s0960-894x(01)00264-5
文献信息
-
Supported Ionic Liquids. New Recyclable Materials for theL-Proline-Catalyzed Aldol Reaction作者:Michelangelo Gruttadauria、Serena Riela、Carmela Aprile、Paolo Lo Meo、Francesca D'Anna、Renato NotoDOI:10.1002/adsc.200505227日期:2006.1New materials for L-proline recycling have been developed. These materials have been applied to the L-proline-catalyzed aldol reaction between acetone and several aldehydes. The L-proline has been supported on the surface of modified silica gels with a monolayer of covalently attached ionic liquid with or without additional adsorbed ionic liquid. Good yields and ee values, comparable with those obtained
-
Imidazolium ion tethered TsDPENs as efficient water-soluble ligands for rhodium catalyzed asymmetric transfer hydrogenation of aromatic ketones作者:Guowei Kang、Silong Lin、Atul Shiwakoti、Bukuo NiDOI:10.1016/j.catcom.2014.08.006日期:2014.12TsDPEN has been synthesized readily and used as a water-soluble ligand for [Cp*RhCl2]2 catalyzed asymmetric transfer hydrogenation (ATH) of aromatic ketones in water. This process provided the secondary alcohols in moderate to excellent conversions (up to 100%) with high enantioselectivities (up to 98% ee) under mild reaction conditions without adding any surfactants. The catalytic system is highly effective
-
Imidazolium-containing diselenides for catalytic oxidations with hydrogen peroxide and sodium bromide in aqueous solutions作者:Eduardo E. Alberto、Antonio L. Braga、Michael R. DettyDOI:10.1016/j.tet.2012.08.004日期:2012.12The design and synthesis of imidazolium-containing diselenides 4a–c is described. The introduction of the N-methylimidazolium group gives freely soluble compounds in water, unlike the majority of common organic diselenides. Catalytic amounts of 4a–c effectively promote bromination of organic substrates using a safe and inexpensive NaBr/H2O2 system in water. Kinetics experiments revealed that the bromination
-
[EN] BICYCLIC INHIBITORS OF HISTONE DEACETYLASE<br/>[FR] INHIBITEURS BICYCLIQUES DE L'HISTONE DÉSACÉTYLASE申请人:RODIN THERAPEUTICS INC公开号:WO2020014602A1公开(公告)日:2020-01-16Provided herein are compounds of Formula I and pharmaceutically acceptable salts and compositions thereof, which are useful for treating a variety of conditions associated with histone deacetylases (HDAC).
-
Synthesis of chiral iron-based ionic liquids: modelling stable hybrid materials作者:Carmen Martin、Israel Cano、Fabio Scé、Rubén Pérez-Aguirre、Carolina Gimbert-Suriñach、Pilar Lopez-Cornejo、Imanol de PedroDOI:10.1039/d0nj00349b日期:——iron-containing ionic liquids were synthetized from several chiral ionic liquids (CILs) based on different imidazolium cations. These novel compounds were prepared through an easy synthetic method involving mixing 1 equivalent of iron salt FeX3 (X = Cl or Br) and 1 equivalent of chiral ionic liquid. The so-obtained chiral iron-based ionic liquids contain easily tunable imidazolium cations, which allow基于不同的咪唑阳离子,由几种手性离子液体(CIL)合成了五种手性铁离子液体。这些新型化合物是通过一种简单的合成方法制备的,该方法包括混合1当量的铁盐FeX 3(X = Cl或Br)和1当量的手性离子液体。如此获得的手性铁基离子液体包含易于调节的咪唑鎓阳离子,其允许在不同的官能团和手性部分中制备多种化合物。这些手性铁基离子液体已通过多种技术进行了全面表征,包括极化测定法,电感耦合等离子体发射光谱法,元素分析,热重分析,差示扫描量热法,磁化率测量以及紫外线可见光,衰减全反射傅立叶变换红外光谱和拉曼光谱。这样的实验证实了这些新材料的结构以及它们的吸引力。IL结构内的手性部分与卤代草酸根阴离子的结合允许形成具有前景的手性,磁性,光学和酸性性质的非常稳定和多功能的化合物。所得的基于手性铁的ILs具有广泛的应用潜力,例如对映选择性催化或手性识别等。
表征谱图
-
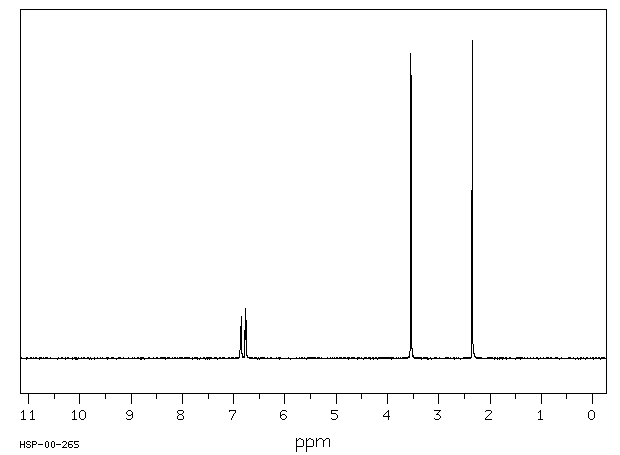
氢谱1HNMR
-
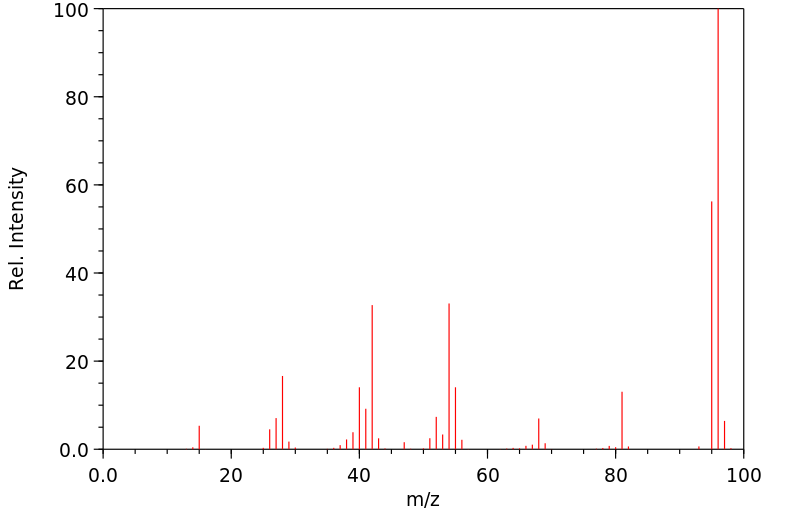
质谱MS
-
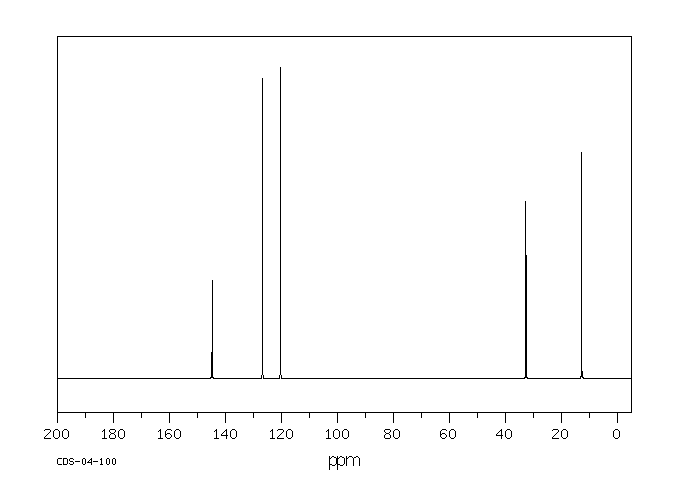
碳谱13CNMR
-
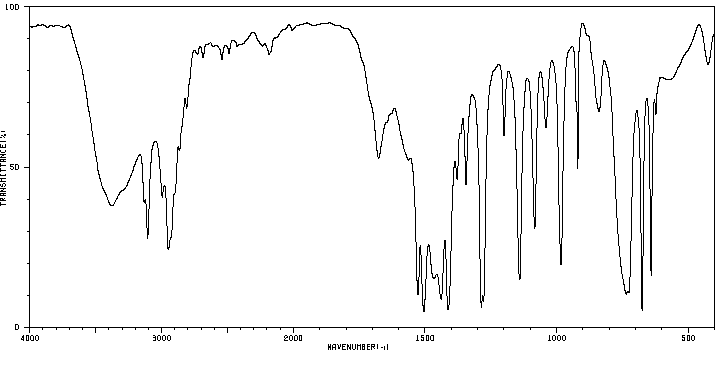
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(SP-4-1)-二氯双(1-苯基-1H-咪唑-κN3)-钯
(5aS,6R,9S,9aR)-5a,6,7,8,9,9a-六氢-6,11,11-三甲基-2-(2,3,4,5,6-五氟苯基)-6,9-甲基-4H-[1,2,4]三唑[3,4-c][1,4]苯并恶嗪四氟硼酸酯
(5-氨基-1,3,4-噻二唑-2-基)甲醇
齐墩果-2,12-二烯[2,3-d]异恶唑-28-酸
黄曲霉毒素H1
高效液相卡套柱
非昔硝唑
非布索坦杂质Z19
非布索坦杂质T
非布索坦杂质K
非布索坦杂质E
非布索坦杂质D
非布索坦杂质67
非布索坦杂质65
非布索坦杂质64
非布索坦杂质61
非布索坦代谢物67M-4
非布索坦代谢物67M-2
非布索坦代谢物 67M-1
非布索坦-D9
非布索坦
非唑拉明
雷非那酮-d7
雷西那德杂质2
雷西纳德杂质L
雷西纳德杂质H
雷西纳德杂质B
雷西纳德
雷西奈德杂质
阿西司特
阿莫奈韦
阿考替胺杂质9
阿米苯唑
阿米特罗13C2,15N2
阿瑞匹坦杂质
阿格列扎
阿扎司特
阿尔吡登
阿塔鲁伦中间体
阿培利司N-1
阿哌沙班杂质26
阿哌沙班杂质15
阿可替尼
阿作莫兰
阿佐塞米
镁(2+)(Z)-4'-羟基-3'-甲氧基肉桂酸酯
锌1,2-二甲基咪唑二氯化物
锌(II)(苯甲醇)(四苯基卟啉)
锌(II)(正丁醇)(四苯基卟啉)
锌(II)(异丁醇)(四苯基卟啉)