1,2-二碘四氟代乙烷 | 354-65-4
中文名称
1,2-二碘四氟代乙烷
中文别名
1,2-二碘四氟乙烷;四氟-1,2-二碘乙烷;1,1,2,2-四氟-1,2-二碘乙烷
英文名称
1,2-diiodotetrafluoroethane
英文别名
1,2-diiodoperfluoroethane;1,1,2,2-tetrafluoro-1,2-diiodoethane
CAS
354-65-4
化学式
C2F4I2
mdl
MFCD00039401
分子量
353.825
InChiKey
NZXVPCQHQVWOFD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-21°C
-
沸点:112-113 °C
-
密度:2.629
-
闪点:112-113°C
-
保留指数:790;791;794
计算性质
-
辛醇/水分配系数(LogP):3.1
-
重原子数:8
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
海关编码:2903799090
-
储存条件:保存条件:室温、密封且干燥环境中。
SDS
| Name: | 1 2-Diiodotetrafluoroethane 98% Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 354-65-4 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 354-65-4 | 1,2-Diiodotetrafluoroethane | 98% | 206-567-2 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.Light sensitive.
Potential Health Effects
Eye:
Causes eye irritation. May cause chemical conjunctivitis.
Skin:
Causes skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. May be harmful if swallowed.
Inhalation:
Causes respiratory tract irritation. May be harmful if inhaled. Can produce delayed pulmonary edema.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas.
Extinguishing Media:
Use foam, dry chemical, or carbon dioxide.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Store protected from light. Use only in a chemical fume hood. Wash clothing before reuse.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Store protected from light.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 354-65-4: Personal Protective Equipment Eyes: Wear chemical splash goggles.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: Not available.
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 112 - 113 deg C
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 2.629
Molecular Formula: C2F4I2
Molecular Weight: 353.8026
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not currently available.
Conditions to Avoid:
Light.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide, hydrogen fluoride gas, hydrogen iodide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 354-65-4 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1,2-Diiodotetrafluoroethane - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 354-65-4: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 354-65-4 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 354-65-4 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 五氟碘乙烷 Pentafluoroethyl iodide 354-64-3 C2F5I 245.919
反应信息
-
作为反应物:描述:参考文献:名称:全氟烷基碘与镉反应中的溶剂效应摘要:全氟有机碘化物(R f I,其中R f = F(CF 3)2 C(CF 2 CF 2)3,nC 6 F 13,nC 8 F 17,F(CF 3)2 COCF 2 CF 2,F之间的相互作用(CF 3)2 CO(CF 2 CF 2)4和C 2 H 5 OC(O)(CF 2 CF 2)与乙腈溶剂介质中的镉混合后,除少量还原产物(R f H)外,主要产生偶联产物(R f R f,产率72-90%)。另一方面,ICF 2 CF 2 I和C1CF 2 CFC1I通过1,2-脱卤反应分别形成烯烃CF 2= CF 2和CF 2= CFC1,作为主要产物。R f I化合物与镉在其他溶剂介质(例如乙醚)中的相互作用。检查了四氢呋喃(THF),N,N-二甲基甲酰胺(DMF)和双(2-甲氧基乙基)醚(二甘醇二甲醚),发现产生不同比例的R fR f和R f H产物。{ft *}当前地址:Fluidics Inc.DOI:10.1016/s0022-1139(00)81020-9
-
作为产物:参考文献:名称:ICF 2 CF 2 I的简便制备及其与乙烯的反应摘要:ICF的一种简便制备2 CF 2我以高收率进行了使用卤素灯。以类似的方式,ICF 2 CF 2我起反应与乙烯,得到ICH 2 CH 2 CF 2 CF 2 CH 2 CH 2 I.DOI:10.1016/s0022-1139(97)00090-0
-
作为试剂:描述:四氟乙烯 、 2,3,4,5-Tetrafluorobenzenethiol 在 1,2-二碘四氟代乙烷 作用下, 反应 0.14h, 生成 perfluoroindane 、 Perfluoro-2,3-dihydrobenzothiophene参考文献:名称:含氟有机硫的化合物。二。全氟-2,3-二氢苯并[ b ]噻吩的合成及其某些化学性质的研究摘要:全氟-2,3-二氢苯并[ b ]噻吩(I)和一些全氟茚满,是通过在碘存在下,将许多4-取代的四氟噻吩酚与四氟乙烯进行共水解而获得的。已经研究了硫化物I与某些亲核试剂和氧化剂的相互作用。已经合成了多氟-2,3-二氢苯并噻吩的5-和6-取代的衍生物。DOI:10.1016/0022-1139(95)03294-n
文献信息
-
The N⋯I Intermolecular Interaction as a General Protocol for the Formation of Perfluorocarbon–Hydrocarbon Supramolecular Architectures 1作者:Paolo Cardillo、Eleonora Corradi、Angelo Lunghi、Stefano Valdo Meille、Maria Teresa Messina、Pierangelo Metrangolo、Giuseppe ResnatiDOI:10.1016/s0040-4020(00)00476-2日期:2000.7The formation of infinite 1D networks where diiodoperfluorocarbons are halogen bonded to di-nitrogen substituted hydrocarbons is described. The N⋯I non-covalent interaction is specific, directional, and strong enough to effectively overcome the low affinity between perfluorocarbon and hydrocarbon modules and to drive their self-assembly in the solid and liquid phase. Several analytical techniques are
-
Studies on fluoroalkylation and fluoroalkoxylation. Part 16. Reactions of fluoroalkyl iodides with some nucleophiles by SRN1 mechanism作者:Qing-Yun Chen、Zai-Ming QiuDOI:10.1016/s0022-1139(00)85017-4日期:1987.3dimer of the anion (). The reaction can be partly suppressed with 1,4-dinitrobenzene(p-DNB) and the radical intermediate can be trapped by diallyl ether (DAE) to give the tetrahydrofuran derivatives. Anions of acetylacetone and malonitrile react also with in the presence of DAE to yield the five-membered ring compounds. All these results seem to indicate that the reaction is a radical chain process氟代烷基碘化物XCF 2 CF 2 I(X = ClCF 2 CF 2,Cl(CF 2)4)()与DMF中的乙酰乙酸乙酯()阴离子容易反应生成()XCF 2 CF 2 H() ,(CH 3 CO)2 CHCO 2 Et()和阴离子的二聚体()。该反应可以用1,4-二硝基苯(p-DNB)部分抑制,自由基中间体可以被二烯丙基醚(DAE)捕获,得到四氢呋喃衍生物。乙酰丙酮和丙二腈的阴离子也会与在DAE的存在下产生五元环化合物。所有这些结果似乎表明该反应是由电子转移引起的自由基链过程。在XCF 2 CF 2 I(X = Cl,I)的情况下,生成四氟乙烯和,而不是和,表明在引发步骤中发生了2-卤代四氟乙基的β片段化。
-
PROCESS FOR PREPARING FLUORINE-CONTAINING ALKOXYALKANE申请人:Nanno Tetsuo公开号:US20080306308A1公开(公告)日:2008-12-11A process for preparing a fluorine-containing alkoxyalkane represented by the general formula (1) R 1 —O—R 2 —O—R 3 where at least one of R 1 , R 2 and R 3 contains one or more fluorine atoms. An alcohol with the highest acidity selected from the group consisting of the compounds represented by the general formula (2) R 1 —OH, the general formula (3) R 3 —O—R 2 —OH, the general formula (4) R 1 —O—R 2 —OH, and the general formula (5) R 3 —OH is reacted with at least one selected from the group consisting of the compounds represented by the general formula (6) Lg-R 2 —O—R 3 , the general formula (7) Lg-R 1 , the general formula (8) Lg-R 3 , and the general formula (9) Lg-R 2 —O—R 1 where Lg represents an anionic leaving group in the presence of a basic compound.一种制备含氟烷氧烷的方法,其通式表示为(1)R1—O—R2—O—R3,其中R1、R2和R3中至少有一个含有一个或多个氟原子。从通式表示的化合物群中选择酸性最高的醇,该化合物群包括通式(2)R1—OH,通式(3)R3—O—R2—OH,通式(4)R1—O—R2—OH和通式(5)R3—OH中的至少一种,与通式表示的化合物群中的至少一种反应,该化合物群包括通式(6)Lg-R2—O—R3,通式(7)Lg-R1,通式(8)Lg-R3和通式(9)Lg-R2—O—R1,其中Lg代表存在碱性化合物的情况下的阴离子离去基团。
-
Stabilizing volatile liquid chemicals using co-crystallization作者:Christer B. Aakeröy、Tharanga K. Wijethunga、Joshua Benton、John DesperDOI:10.1039/c4cc09650a日期:——
A convenient, effective, and scalable protocol for stabilizing volatile liquid chemicals is reported.
报道了一种方便、有效和可扩展的协议,用于稳定挥发性液态化学品。 -
Electrostatic Potential Differences and Halogen-Bond Selectivity作者:Christer B. Aakeröy、Tharanga K. Wijethunga、John Desper、Marijana ĐakovićDOI:10.1021/acs.cgd.5b01770日期:2016.5.4kJ/mol, and in all of the co-crystals found herein (7/7), the halogen-bond donor favored the best acceptor site. These results allow us to propose some tentative guidelines and rationales for halogen-bond preferences in competitive systems. If ΔE < 35 kJ/mol, the electrostatic potential difference is not large enough to allow the donor molecules to form halogen bonds of sufficiently different thermodynamic通过对9个全氟化卤素键供体和12个对位受体进行系统性共结晶,探讨了基于分子静电势的卤素键相互作用选择性的指南,该两个单体呈现出两个具有不同静电势的结合位点。108个反应中总共89个导致共晶形成(如IR光谱所示),获得了35个新的晶体结构。为了避免在这些实验中出现任何潜在的溶剂-溶质偏差,甲醇专门用作晶体生长的溶剂。根据每种情况下观察到的卤素键连接性的具体性质,将结构分为三个不同的组。每个分子上两个受体位点之间的静电势差定义为ΔE值。第1组包含ΔE值低于35kJ / mol单位的受体分子,并且在该类别中,卤素键发生在所有共晶体(9/9)的两个结合位点上。第2组中的对位受体分子的特征在于ΔE值在35–65 kJ / mol范围内,在该组中,一半的结构显示卤素键与最佳受体(11/22),一半的结构显示卤素键与最佳受体。两个结合位点(11/22)。在第3组中,ΔE值> 167kJ / mol,并且
表征谱图
-
氢谱1HNMR
-
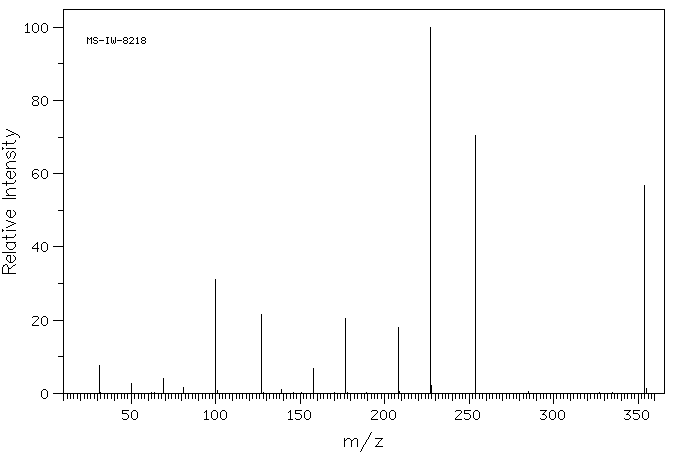
质谱MS
-
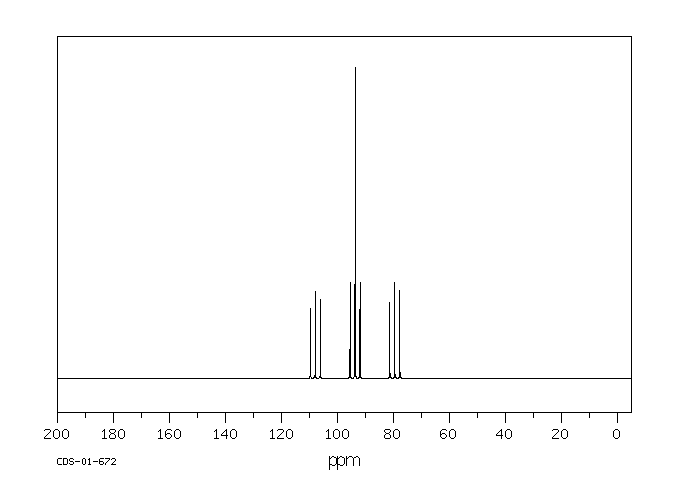
碳谱13CNMR
-
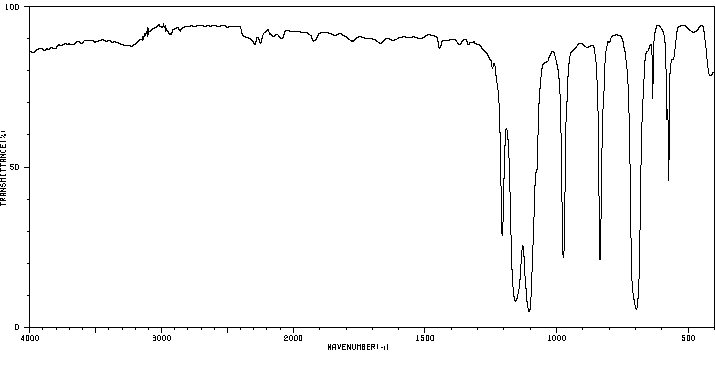
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
胍,N-[3-(氨基甲基)-5-甲基苯基]-N'-乙基-
碘甲烷
碘甲基环辛烷
碘甲基环戊烷
碘环庚烷
碘环十二烷
碘环丁烷
碘十六烷
碘代环戊烷
碘代正辛烷-D2
碘代异丁烷
碘代叔丁烷
碘代丙烷-D7
碘代丙烷-D3
碘代丙烷-D2
碘代丙烷-D2
碘乙烷-d<
碘乙烷-D1
碘乙烷-2-13C
碘乙烷-2,2,2-d3
碘乙烷-1-13C
碘乙烷-1,1-d2
碘乙烷(1,2-13C2)
碘乙烷
碘丁烷-D9
碘(碘甲氧基)甲烷
甲基碘化钙
环辛烷,1-氟-2-碘-,反-
环戊二烯并[1,3]环丙烯并[1,2]环庚烯-2(1H)-酮,八氢-3a,5,5-三甲基-,(3aR,3bR,8aS)-rel-
环丙基碘
无花果蛋白酶来源于无花果树乳胶
新戊氧基
新戊基碘
抗-8-碘-1,5-二甲基二环<3.2.1>辛烷
抗-8-碘-1,5-二甲基二环<3.2.1>辛烷
异戊基碘
异丁基锰(II)碘化物
反式-4-己烯基碘
十氢-2-(碘甲基)-萘
十四烷基碘化物
十五氟碘庚烷
十九氟-9-碘壬烷
全氟辛基碘烷
全氟碘代丁烷
全氟异戊基碘
全氟异庚基碘化物
全氟异壬基碘
全氟异十一烷基碘化物
全氟己基碘烷
全氟叔丁基碘化物