1,3,3-三苯基-1-丙酮 | 606-86-0
分子结构分类
中文名称
1,3,3-三苯基-1-丙酮
中文别名
——
英文名称
1,3,3-triphenylpropan-1-one
英文别名
1,3,3-triphenyl-1-propanone;3,3-Diphenylpropiophenone
CAS
606-86-0
化学式
C21H18O
mdl
——
分子量
286.373
InChiKey
WYRBITQXPQGTBL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:96.0 °C
计算性质
-
辛醇/水分配系数(LogP):4.9
-
重原子数:22
-
可旋转键数:5
-
环数:3.0
-
sp3杂化的碳原子比例:0.1
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
海关编码:2914399090
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product identifiers
Product name : Propiophenone, 3,3-diphenyl-
CAS-No. : 606-86-0
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008 [EU-GHS/CLP]
Eye irritation (Category 2)
Chronic aquatic toxicity (Category 4)
This substance is not classified as dangerous according to Directive 67/548/EEC.
Label elements
Labelling according Regulation (EC) No 1272/2008 [CLP]
Pictogram
Signal word Warning
Hazard statement(s)
H319 Causes serious eye irritation.
H413 May cause long lasting harmful effects to aquatic life.
Precautionary statement(s)
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove
contact lenses, if present and easy to do. Continue rinsing.
Supplemental Hazard none
Statements
Other hazards - none
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Formula : C21H18O
Molecular Weight : 286,37 g/mol
Section 4. FIRST AID MEASURES
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Consult a physician.
In case of eye contact
Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.
Most important symptoms and effects, both acute and delayed
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Indication of any immediate medical attention and special treatment needed
no data available
Section 5. FIREFIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid dust formation. Avoid breathing vapors, mist or gas. Ensure
adequate ventilation. Avoid breathing dust.
Environmental precautions
Prevent further leakage or spillage if safe to do so. Do not let product enter drains. Discharge into the
environment must be avoided.
Methods and materials for containment and cleaning up
Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Avoid contact with skin and eyes. Avoid formation of dust and aerosols.
Provide appropriate exhaust ventilation at places where dust is formed.Normal measures for preventive fire
protection.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Specific end uses
no data available
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and
at the end of workday.
Personal protective equipment
Eye/face protection
Safety glasses with side-shields conforming to EN166 Use equipment for eye protection tested
and approved under appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
impervious clothing, The type of protective equipment must be selected according to the
concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
For nuisance exposures use type P95 (US) or type P1 (EU EN 143) particle respirator.For higher
level protection use type OV/AG/P99 (US) or type ABEK-P2 (EU EN 143) respirator cartridges.
Use respirators and components tested and approved under appropriate government standards
such as NIOSH (US) or CEN (EU).
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
a) Appearance Form: solid
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing no data available
point
f) Initial boiling point and no data available
boiling range
g) Flash point no data available
h) Evaporation rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility no data available
o) Partition coefficient: n- log Pow: 4,943
octanol/water
p) Autoignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
Section 10. STABILITY AND REACTIVITY
Reactivity
no data available
Chemical stability
no data available
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
Section 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Potential health effects
Inhalation May be harmful if inhaled. May cause respiratory tract irritation.
Ingestion May be harmful if swallowed.
Skin May be harmful if absorbed through skin. May cause skin irritation.
Eyes
Causes eye irritation.
Signs and Symptoms of Exposure
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Additional Information
RTECS: UH1588150
Section 12. ECOLOGICAL INFORMATION
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
no data available
Other adverse effects
no data available
Section 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed
professional waste disposal service to dispose of this material. Dissolve or mix the material with a
combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
Section 15. REGULATORY INFORMATION
This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.
Safety, health and environmental regulations/legislation specific for the substance or mixture
no data available
Chemical Safety Assessment
no data available
Section 16. OTHER INFORMATION
Further information
Copyright 2012 Co. LLC. License granted to make unlimited paper copies for internal use
only.
The above information is believed to be correct but does not purport to be all inclusive and shall be
used only as a guide. The information in this document is based on the present state of our knowledge
and is applicable to the product with regard to appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Corporation and its Affiliates shall not be held
liable for any damage resulting from handling or from contact with the above product. See
and/or the reverse side of invoice or packing slip for additional terms and conditions of sale.
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-二苯甲基-1,3-二苯基丙烷-1,3-二酮 2-benzhydryl-1,3-diphenylpropane-1,3-dione 60999-93-1 C28H22O2 390.481 2-溴-1,3,3-三苯基-1-丙酮 2-bromo-1,3,3-triphenyl-1-propanone 106552-33-4 C21H17BrO 365.269 2-二苯甲基-1-苯基丁烷-1,3-二酮 2-benzhydryl-1-phenylbutane-1,3-dione 33925-42-7 C23H20O2 328.411 ω-苄基苯乙酮 dihydrochalcone 1083-30-3 C15H14O 210.276 二苯甲酰基甲烷 1,3-diphenylpropanedione 120-46-7 C15H12O2 224.259 —— β-Bis(p-chlorphenyl)propionylchlorid 55010-16-7 C15H11Cl3O 313.611 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1,4,4-三苯基-2,2-二甲基-1-丙酮 1,4,4-triphenyl-2,2-dimethyl-1-propanone 126256-02-8 C23H22O 314.427 2-溴-1,3,3-三苯基-1-丙酮 2-bromo-1,3,3-triphenyl-1-propanone 106552-33-4 C21H17BrO 365.269 3-羟基-1,3,3-三苯基-丙-1-酮 3-hydroxy-1,3,3-triphenyl-propan-1-one 6624-02-8 C21H18O2 302.373 2-二苯甲基-1-苯基丁烷-1,3-二酮 2-benzhydryl-1-phenylbutane-1,3-dione 33925-42-7 C23H20O2 328.411 1,4-二苯丁烷 1,1-diphenylbutane 719-79-9 C16H18 210.319 —— 1,1-diphenyl-2-methylpropane 1634-11-3 C16H18 210.319 —— 1,3,3-triphenylpropan-1-ol 70550-49-1 C21H20O 288.389 —— 2-acetyloxy-1,3,3-triphenyl-1-propanone —— C23H20O3 344.41 —— 1,1,1-trifluoro-4,4-diphenylbutan-2-one —— C16H13F3O 278.274 2,2-二甲基-3,3-二苯基-丙酸酰胺 2,2-dimethyl-3,3-diphenyl-propionic acid amide 861363-09-9 C17H19NO 253.344
反应信息
-
作为反应物:描述:参考文献:名称:Albesco, Annales de Chimie (Cachan, France), 1922, vol. <9> 18, p. 221摘要:DOI:
-
作为产物:描述:参考文献:名称:羰基稳定的化钠与格氏试剂的反应摘要:如果R''或R'''具有α-氢,则二酰基甲磺酸RRRR'-;(COR')2与R''MgX反应生成酮R'COR'''。在没有这种情况的情况下,例如,R(Me)(COPh)2与PhMgBr的反应导致S-; Me的消除,得到产物RSC(COPh 2)。 '”和R'''在所有pH团,二苯甲酮且Ph 2 HCOPh形成。phenacylides R(Me)的HCOPh与R'''MGX反应消去R'''我的产生RSCH前进2 COPH。与PhLi的反应这些叶立德也被记录下来。DOI:10.1016/s0040-4020(01)82900-8
文献信息
-
Visible-Light-Promoted Photocatalyst-Free Hydroacylation and Diacylation of Alkenes Tuned by NiCl<sub>2</sub>·DME作者:Xinxin Zhao、Bing Li、Wujiong XiaDOI:10.1021/acs.orglett.9b04595日期:2020.2.74-dihydropyridines via an acyl radical addition and hydrogen atom transfer pathway under photocatalyst-free conditions. The efficiency was highlighted by wide substrate scope, good to high yields, successful scale-up experiments, and expedient preparation of highly functionalized ketone derivatives. In addition, this protocol allows for the synthesis of 1,4-dicarbonyl compounds through alkene diacylation in the presence
-
9-(Diphenylphosphino)anthracene-based phosphapalladacycle catalyzed conjugate addition of arylboronic acids to electron-deficient alkenes作者:Minori Shimizu、Tetsuya YamamotoDOI:10.1016/j.tetlet.2020.152257日期:2020.89-(Diphenylphosphino)anthracene-based phosphapalladacycle catalyzed conjugate addition of arylboronic acids to electron-deficient alkenes such asα,β-unsaturated ketones, esters, nitrile and nitroalkenes gave corresponding β-arylated alkanes in good yields and achieved TON up to 700.
-
Heterogeneous catalytic 1,4-addition of arylmagnesium compounds to chalcones作者:Kinga Juhász、Zoltán HellDOI:10.1016/j.tetlet.2018.07.016日期:2018.8Copper(II) on a 4 Å molecular sieve support catalyses the chemoselective addition of alkyl- and arylmagnesium halides to chalcones. Only the 1,4-addition products were obtained in high yields.
-
Uncatalyzed conjugate addition of organozinc halides to enones in DME: a combined experimental/computational study on the role of the solvent and the reaction mechanism作者:Gianluca Casotti、Gianluca Ciancaleoni、Filippo Lipparini、Chiara Nieri、Anna IulianoDOI:10.1039/c9sc04820k日期:——calculations, prompted by the experimental aggregation study, revealed an unexpected reaction mechanism, where the coordinating capabilities of DME stabilize a transition state involving two organozinc moieties, lowering the activation energy of the reaction with respect to that seen for THF, enough to explain the fast and quantitative reactions observed experimentally and the different behaviors of
-
Direct functionalization of benzylic C–Hs with vinyl acetates via Fe-catalysis作者:Chun-Xiao Song、Gui-Xin Cai、Thomas R. Farrell、Zhong-Ping Jiang、Hu Li、Liang-Bing Gan、Zhang-Jie ShiDOI:10.1039/b911031c日期:——Direct cross-coupling to construct sp3 Câsp3C bonds viaFe-catalyzed benzylic CâH activation with 1-aryl vinyl acetate was developed.
表征谱图
-
氢谱1HNMR
-
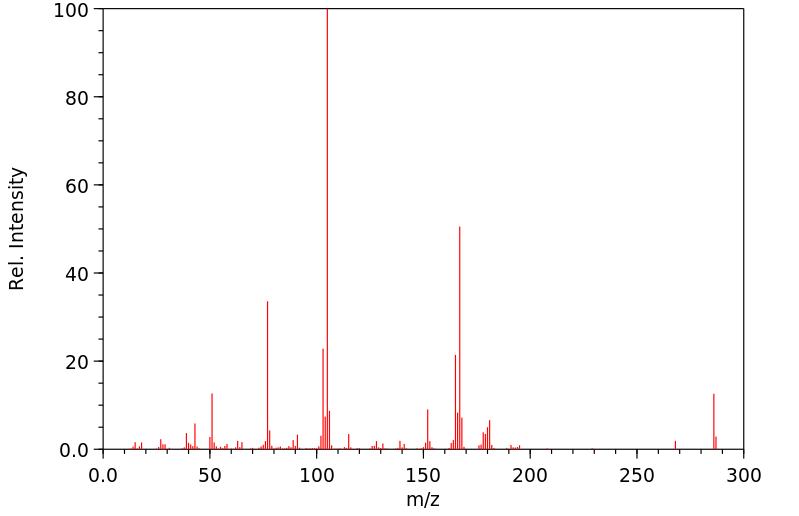
质谱MS
-
碳谱13CNMR
-
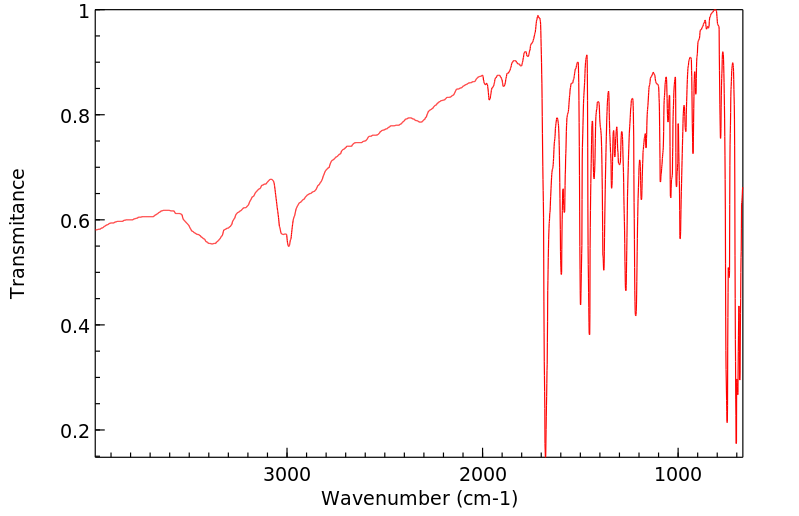
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2Z)-1,3-二苯基-2-丙烯-1-酮,2-丙烯-1-酮,1,3-二苯基-,(2Z)-
龙血素D
龙血素A
龙血素 B
黄色当归醇F
黄色当归醇B
黄腐醇; 黄腐酚
黄腐醇 D; 黄腐酚 D
黄腐酚B
黄腐酚
黄腐酚
黄卡瓦胡椒素 C
高紫柳查尔酮
阿普非农
阿司巴汀
阿伏苯宗
金鸡菊查耳酮
邻肉桂酰苯甲酸
达泊西汀杂质25
豆蔻明
补骨脂色烯查耳酮
补骨脂查耳酮
补骨脂呋喃查耳酮
补骨脂乙素
蜡菊亭; 4,2',4'-三羟基-6'-甲氧基查耳酮
苯酚,4-[3-(2-羟基苯基)-1-苯基丙基]-2-(3-苯基丙基)-
苯磺酰胺,N-[4-[3-(3-羟基苯基)-1-羰基-2-丙烯基]苯基]-
苯磺酰胺,N-[3-[3-(4-羟基-3-甲氧苯基)-1-羰基-2-丙烯基]苯基]-
苯磺酰胺,4-甲氧基-N,N-二甲基-2-(3-羰基-3-苯基-1-丙烯基)-,(E)-
苯磺酰氯化,4,5-二甲氧基-2-(3-羰基-3-苯基-1-丙烯基)-,(E)-
苯磺酰氯,4-甲氧基-3-(3-羰基-3-苯基-1-丙烯基)-,(E)-
苯甲醇,4-甲氧基-a-[2-(4-甲氧苯基)乙烯基]-
苯甲酸-[4-(3-氧代-3-苯基-丙烯基)-苯胺]
苯甲酸,3-[3-(4-溴苯基)-1-羰基-2-丙烯基]-4-羟基-
苯甲酰(2-羟基苯酰)甲烷
苯甲腈,4-(1-羟基-3-羰基-3-苯基丙基)-
苯基[2-(1-萘基)乙烯基]甲酮
苯基-(三苯基-丙-2-炔基)-醚
苯基-(2-苯基-2,3-二氢-苯并噻唑-2-基)-甲酮
苯亚甲基苯乙酮
苯乙酰腈,a-(1-氨基-2-苯基亚乙基)-
苯丙酸,a-苯甲酰-b-羰基-,苯基(苯基亚甲基)酰肼
苯,1-(2,2-二甲基-3-苯基丙基)-2-甲基-
苏木查耳酮
苄桂哌酯
苄基(4-氯-2-(3-氧代-1,3-二苯基丙基)苯基)氨基甲酸酯
芦荟提取物
腈苯唑
胀果甘草宁C
聚磷酸根皮酚