1,3,5-三甲氧基-2-硝基苯 | 14227-18-0
中文名称
1,3,5-三甲氧基-2-硝基苯
中文别名
2,4,6-三甲氧基硝基苯
英文名称
1,3,5-trimethoxy-2-nitrobenzene
英文别名
2,4,6-trimethoxy nitrobenzene;2,4,6-trimethoxynitrobenzene;1,3,5-trimethoxy-2-nitro-benzene;1,3,5-Trimethoxy-2-nitro-benzol;Nitrophloroglucin-trimethylaether;4-nitro-1,3,5-trimethoxybenzene
CAS
14227-18-0
化学式
C9H11NO5
mdl
MFCD00016992
分子量
213.19
InChiKey
VWYAWLZEMLQGJH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:149-151 °C
-
沸点:353.17°C (rough estimate)
-
密度:1.3707 (rough estimate)
-
稳定性/保质期:
在常温常压下稳定,应避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:15
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:73.5
-
氢给体数:0
-
氢受体数:5
安全信息
-
危险品标志:Xi
-
安全说明:S24/25
-
危险性防范说明:P280,P305+P351+P338
-
危险性描述:H317,H319
SDS
| Name: | 2 4 6-Trimethoxynitrobenzene Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 14227-18-0 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 14227-18-0 | 2,4,6-Trimethoxynitrobenzene | 99 | unlisted |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated. May be harmful if swallowed.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. May be harmful if inhaled.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Get medical aid.
Skin:
In case of contact, flush skin with plenty of water. Remove contaminated clothing and shoes. Get medical aid if irritation develops and persists. Wash clothing before reuse.
Ingestion:
If swallowed, do not induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation:
If inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation.
Minimize dust generation and accumulation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing.
Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 14227-18-0: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystalline powder
Color: yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 149-151 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C9H11NO5
Molecular Weight: 213.19
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Dust generation.
Incompatibilities with Other Materials:
Strong oxidizing agents, strong bases.
Hazardous Decomposition Products:
Hydrogen cyanide, carbon monoxide, oxides of nitrogen, carbon dioxide, nitric acid.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 14227-18-0 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2,4,6-Trimethoxynitrobenzene - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
IMO
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 14227-18-0: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 14227-18-0 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 14227-18-0 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-硝基间苯三酚 1,3,5-Trihydroxy-2-nitrobenzene 16600-92-3 C6H5NO5 171.109 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3,5-Dimethoxy-2-nitro-phenol 16600-87-6 C8H9NO5 199.163 1,3,5-三甲氧基-2,4-二硝基苯 1,3,5-trimethoxy-2,4-dinitro-benzene 18523-15-4 C9H10N2O7 258.188 2,4,6-三甲氧基苯胺 2,4,6-trimethoxyaniline 14227-17-9 C9H13NO3 183.207
反应信息
-
作为反应物:描述:参考文献:名称:旋光镍(II)配合物催化非手性异氰化物的螺杆选择性聚合摘要:各种非手性异氰化物与该催化剂的聚合产生光学活性聚合物,其对映体过量高达 83%。在质子酸、路易斯酸或 Ni(II) 盐作为催化剂存在下,异氰化物聚合生成聚(异氰化物),也称为聚(亚氨基亚甲基)或聚(碳酰亚胺酰基)。1,2 Ni(II) 盐是多功能催化剂,在我们看来,最适合我们的实验。聚(异氰化物)是方案 I n Ni(II) ( RN = C < ) 中的不寻常聚合物 „ 其主链的每个原子都带有侧链 .1,2 这一特征导致围绕单键的旋转受限连接主链碳原子。单键周围可能有两种构象,即。R 和 S 。所得聚合物将是具有P和M螺杆的非对映异构分子的混合物。这种混合物将包含过量的一种螺旋感。在大约 20 种不同的光学活性异氰化物 0.10" 12 中观察到了这一点。在另一个过程中,我们通过特异性抑制外消旋对的一个螺旋方向的生长来制备光学活性聚合物。5,13 最后,在一种情况下,光学活性聚合物活性聚DOI:10.1021/ja00228a035
-
作为产物:描述:参考文献:名称:卤化物钙钛矿纳米晶体上成对氧化还原位点之间的可调谐光催化双电子穿梭摘要:钙钛矿半导体作为先进的太阳能转换材料是用于光氧化还原有机合成的有前途的催化剂。尽管钙钛矿表面产生了高浓度的电荷载流子,但有效利用这些非平衡和杂乱的高能载流子来触发化学反应仍然是一个热门且具有挑战性的课题。在这里,我们报告了钙钛矿纳米晶体上成对氧化还原位点之间的光子介导电子穿梭,用于重新形成高度稳定的碳-卤素键,同时利用表面电子和空穴。通过精确控制表面还原/氧化反应速率,可以毫不费力地调整光氧化还原级联反应,从而解锁各种(杂)芳烃的转化。DOI:10.1021/acscatal.2c01044
文献信息
-
Synthesis, Molecular Structure, Fluxional Behavior, and Tricarbonyliron Transfer Reactions of (η4-1-Azabuta-1,3-diene)tricarbonyliron Complexes作者:Hans-Joachim Knölker、Gerhard Baum、Norbert Foitzik、Helmut Goesmann、Peter Gonser、Peter G. Jones、Herbert RötteleDOI:10.1002/(sici)1099-0682(199807)1998:7<993::aid-ejic993>3.0.co;2-6日期:1998.7(η4-1-azabuta-1,3-diene)tricarbonyliron complexes 10 are easily prepared in high yield by condensation of the corresponding arylamines 7 with the cinnamaldehydes 8 and subsequent ultrasound-promoted complexation of the resulting 1-azabuta-1,3-dienes 9 with nonacarbonyldiiron. The complexes 10 are shown to represent excellent reagents for the transfer of the tricarbonyliron fragment onto cyclohexa-1(η4-1-azabuta-1,3-diene)tricarbonyliron 配合物 10 很容易通过相应的芳胺 7 与肉桂醛 8 的缩合和随后产生的 1-azabuta-1,3 的超声促进络合以高产率制备-二烯 9 与九羰基二铁。显示复合物 10 代表了用于将三羰基铁片段转移到环己-1,3-二烯 (1a) 上的极好试剂。配合物 10 的结构表征是通过 IR、1H-NMR 和 13C-NMR 光谱以及 10b、10c 和 10l 的 X 射线晶体学来实现的。使用变温 13C-NMR 光谱研究了配合物 10a、10b、10c、10e 和 2 的流动性,并确定了三羰基铁碎片旋转门旋转的激活势垒。
-
Highly selective reduction of nitrobenzenes to azoxybenzenes with a copper catalyst作者:Zhichao Chen、Yatao Qiu、Xiaoxing Wu、Yong Ni、Li Shen、Jun Wu、Sheng JiangDOI:10.1016/j.tetlet.2018.02.064日期:2018.4A convenient protocol for highly selective delivery of azoxybenzenes from reduction of nitrobenzenes was developed by utilizing a copper catalyst. A variety of functional groups and substitution were well tolerated.
-
一种2,4,6-三甲氧基硝基苯的制备方法申请人:黑龙江格林赫思生物科技有限公司公开号:CN104387276B公开(公告)日:2016-08-17
-
Aromatic nitration with ion radical pairs [ArH.cntdot.+,NO2.cntdot.] as reactive intermediates. Time-resolved studies of charge-transfer activation of dialkoxybenzenes作者:S. Sankararaman、W. A. Haney、Jay K. KochiDOI:10.1021/ja00251a032日期:1987.8examined in the substituted p-dimethoxybenzenes CH/sub 3/OC/sub 6/H/sub 4/OCH/sub 2/X with X = CO/sub 2/H, CO/sub 2//sup -/, CO/sub 2/Et, and CH/sub 2/OH as the pendant functional groups. The mechanistic relevance of the collapse of (ArH/sup +/, NO/sub 2/) to the Wheland intermediate is discussed in the context of electrophilic aromatic nitrations.在亲电条件下和通过电荷转移活化进行的芳族硝化作用提供相同的产率和来自常见系列芳族醚 (ArH) 的硝化产物的异构体分布。时间分辨光谱确定电荷转移硝化通过离子自由基对 (ArH/sup +/,NO/sub 2/) 进行,由电子供体 - 受体或 ..pi.. 复合物的有意激发产生芳烃与 C(NO/sub 2/)/sub 4/。电荷转移带的激光闪光光解定义了芳烃阳离子自由基 ArH/sup +/ 的演化,并允许在各种溶剂和添加的盐中描绘其衰变动力学。在取代的对二甲氧基苯 CH/sub 3/OC/sub 6/H/sub 4/OCH/sub 2/X 中检查 ArH/sup +/ 的内部捕获,其中 X = CO/sub 2/H, CO/ sub 2//sup -/, CO/sub 2/Et, 和 CH/sub 2/OH 作为侧基官能团。在亲电芳香硝化的背景下讨论了 (ArH/sup +/, NO/sub
-
Assembly of Primary (Hetero)Arylamines via CuI/Oxalic Diamide-Catalyzed Coupling of Aryl Chlorides and Ammonia作者:Mengyang Fan、Wei Zhou、Yongwen Jiang、Dawei MaDOI:10.1021/acs.orglett.5b03230日期:2015.12.4A general and practical catalytic system for aryl amination of aryl chlorides with aqueous or gaseous ammonia has been developed, with CuI as the catalyst and bisaryl oxalic diamides as the ligands. The reaction proceeds at 105–120 °C to provide a diverse set of primary (hetero)aryl amines in high yields with various functional groups.
表征谱图
-
氢谱1HNMR
-
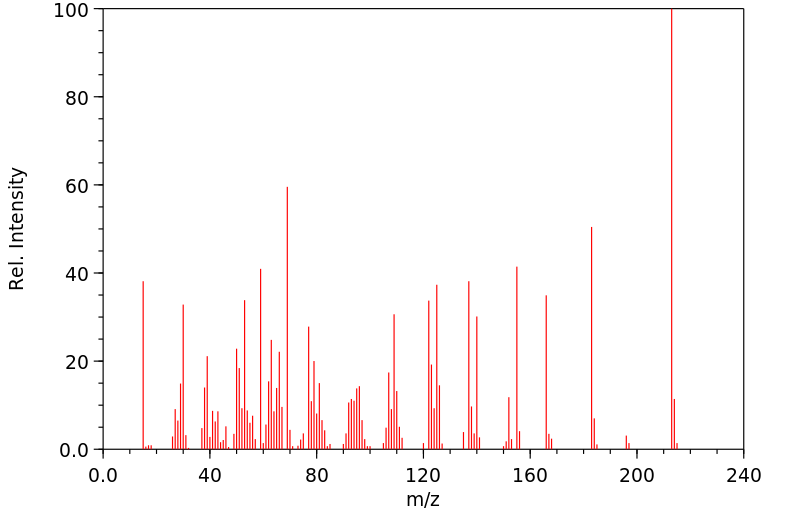
质谱MS
-
碳谱13CNMR
-
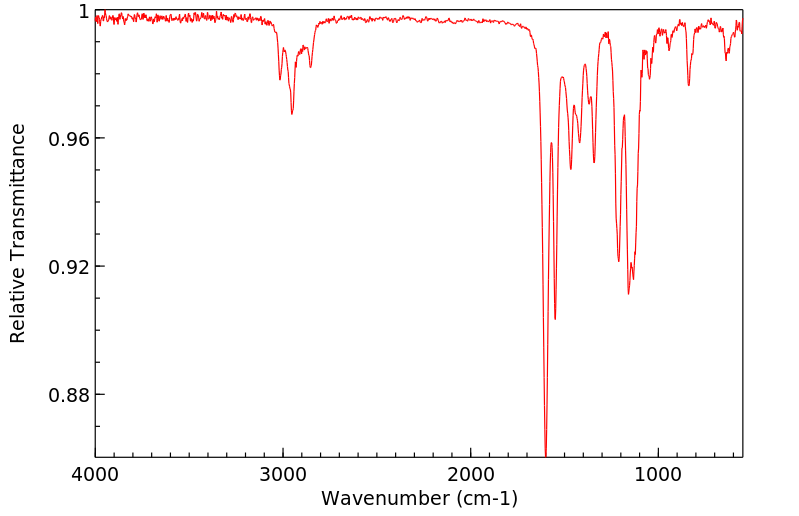
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫