1,3-二氨基-2-丙醇-N,N,N,N-四乙酸 | 3148-72-9
物质功能分类
中文名称
1,3-二氨基-2-丙醇-N,N,N,N-四乙酸
中文别名
N,N'-(2-羟基-1,3-丙二基)双[N-(羧甲基)]甘氨酸;1,3-二氨基-2-丙醇-N,N,N',N'-四乙酸;1,3-二氨-2-羟丙烷-N,N,N',N'-四乙酸
英文名称
1,3-diamino-2-propanol-N,N,N',N'-tetraacetic acid
英文别名
2-hydroxytrimethylenediaminetetra(acetic acid);1,3-diamino-2-hydroxypropane-N,N,N′,N′-tetraacetic acid;Dhpta;2-[[3-[bis(carboxymethyl)amino]-2-hydroxypropyl]-(carboxymethyl)amino]acetic acid
CAS
3148-72-9
化学式
C11H18N2O9
mdl
MFCD00004288
分子量
322.272
InChiKey
WYMDDFRYORANCC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:194 °C (dec.)(lit.)
-
沸点:460.9°C (rough estimate)
-
密度:1.3839 (rough estimate)
-
溶解度:在1mol/L NaOH中几乎完全溶解
-
稳定性/保质期:
遵照规定使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):-6.5
-
重原子数:22
-
可旋转键数:12
-
环数:0.0
-
sp3杂化的碳原子比例:0.636
-
拓扑面积:176
-
氢给体数:5
-
氢受体数:11
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
危险类别码:R36
-
WGK Germany:2
-
RTECS号:AI2930000
-
海关编码:2922509090
-
安全说明:S26,S36
-
危险标志:GHS07
-
危险性描述:H315,H319,H335
-
危险性防范说明:P261,P305 + P351 + P338
-
储存条件:应将密闭存储在阴凉干燥的环境中。
SDS
1,3-二氨基-2-丙醇-N,N,N',N'-四乙酸 修改号码:2
模块 1. 化学品
产品名称: 1,3-Diamino-2-propanol-N,N,N',N'-tetraacetic Acid
修改号码: 2
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 1,3-二氨基-2-丙醇-N,N,N',N'-四乙酸
百分比: >98.0%(T)
CAS编码: 3148-72-9
俗名: DPTA-OH
分子式: C11H18N2O9
1,3-二氨基-2-丙醇-N,N,N',N'-四乙酸 修改号码:2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色类白色
气味: 无资料
1,3-二氨基-2-丙醇-N,N,N',N'-四乙酸 修改号码:2
模块 9. 理化特性
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度: 无资料
模块 10. 稳定性和反应性
稳定性: 一般情况下稳定。
反应性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: ipr-rat LDLo:800 mg/kg
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
RTECS 号码: AI2930000
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
1,3-二氨基-2-丙醇-N,N,N',N'-四乙酸 修改号码:2
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布): 针对危险化学品的安全使用、生产、储存、运输、装
卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 1,3-Diamino-2-propanol-N,N,N',N'-tetraacetic Acid
修改号码: 2
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 1,3-二氨基-2-丙醇-N,N,N',N'-四乙酸
百分比: >98.0%(T)
CAS编码: 3148-72-9
俗名: DPTA-OH
分子式: C11H18N2O9
1,3-二氨基-2-丙醇-N,N,N',N'-四乙酸 修改号码:2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色类白色
气味: 无资料
1,3-二氨基-2-丙醇-N,N,N',N'-四乙酸 修改号码:2
模块 9. 理化特性
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度: 无资料
模块 10. 稳定性和反应性
稳定性: 一般情况下稳定。
反应性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: ipr-rat LDLo:800 mg/kg
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
RTECS 号码: AI2930000
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
1,3-二氨基-2-丙醇-N,N,N',N'-四乙酸 修改号码:2
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布): 针对危险化学品的安全使用、生产、储存、运输、装
卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
制备方法与用途
用途:螯合剂。
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— tetramethyl <(2-hydroxytrimethylene)dinitrilo>tetrakisacetate 87218-62-0 C15H26N2O9 378.379
反应信息
-
作为反应物:描述:1,3-二氨基-2-丙醇-N,N,N,N-四乙酸 在 氯化亚砜 作用下, 以 甲醇 为溶剂, 反应 144.0h, 生成 26-hydroxy-3,11,15,23-tetraoxo-1,4,7,10,13,16,19,22-octaazabicyclo[11.11.3]heptacosane参考文献:名称:Novel Properties of Cooperative Dinuclear Zinc(II) Ions: The Selective Recognition of Phosphomonoesters and Their P−O Ester Bond Cleavage by a New Dinuclear Zinc(II) Cryptate摘要:A propanol-bridged octaazacryptand (26-hydroxy-1,4,7,10,13,16,19,22-octaazabicyclo [11.11.3]heptacosane, HL) has been synthesized from diethylenetriamine and [2-oxo-6-(aminomethyl)morpholyl]-N,N',N'-triacetic acid triethyl ester by refluxing in MeOH followed by BH3 . THF reduction. This octaazacryptand forms a novel dinuclear zinc(II) cryptate (Zn(2)L) (L = alkoxide form of HL) in aqueous solution. The X-ray crystal structure of the cryptate showed each zinc(II) ion in a distorted trigonal-bipyramidal environment involving two NH's and an alkoxide O- anion as equatorial donors, with tertiary amine and an NH stand in apical positions. Crystals of the triperchlorate salt of Zn(2)L (C19H43N8O13Cl3Zn2) are monoclinic, space group P2(1)/n (No. 14) with a = 15.037(5) Angstrom, b = 13.862(5) Angstrom, c = 15.780(4) Angstrom, beta = 90.29(2)degrees, Z = 4, and R = 0.109. Although the two zinc(II) ions (separated with a distance of 3.42 Angstrom) in the cryptate appeared to be coordinately saturated and hence were assumed unreactive, they work together to selectively interact with a phosphomonoester, 4-nitrophenyl phosphate dianion (NPP2-), and promote the cleavage of its P-O ester bond by nucleophilic attack of one of the apically coordinated NH's at pH 4.9-9.5 in aqueous solution. The reaction product was isolated as a phosphoramide derivative (Zn(2)L-PO3H) from aqueous solution at pH 3 and characterized by X-ray crystal analysis. Crystals of Zn(2)L-PO3H . 2H(2)O .(ClO4)(3)(C19H48N8O18Cl3Zn2) are monoclinic, space group Cc (No. 9) with a = 19.573(3) Angstrom, b = 12.454(4) Angstrom, c = 15.066(3) Angstrom, beta = 103.94(1)degrees, Z = 4, and R = 0.036. The structure of Zn(2)L-PO3H featured the two phosphoryl oxygens bound to each zinc(II) ion in place of the original two apical NH's. The kinetics were followed for liberation of 4-nitrophenol (the maximum second-order rate constant k(NPP) = (1.52 +/- 0.05) x 10(-3) M(-1) s(-1) at pH 5.9 and 35 degrees C). The rate vs pH profile with 5 mM Zn(2)L and 10 mM 4-nitrophenyl phosphate showed a bell-shaped curve with pK(2) of 5.2 and pK(2) of 6.3, which were assigned to the protonation constants for NPP2- + H reversible arrow HNPP- and NH (a freed apical donor in the associated reaction intermediate) + H+ reversible arrow HNH+, respectively. The most remarkable character of the present new zinc(II) cryptate was that it reacted only with phosphoester polyanions such as NPP2- and ATP(4-), but not with bis(4- nitrophenyl) phosphodiester monoanion, tris(4-nitrophenyl phosphotriester, or 4-nitrophenyl acetate. The present results may well be relevant to the significance of dinuclear metal centers in metallophosphatases such as alkaline monophosphatase.DOI:10.1021/ja953413m
-
作为产物:描述:4,4'-(2-hydroxypropane-1,3-diyl)bis(piperazine-2,6-dione) 在 sodium hydroxide 作用下, 以 水 为溶剂, 反应 24.0h, 生成 1,3-二氨基-2-丙醇-N,N,N,N-四乙酸参考文献:名称:右雷佐生类似物的构效关系研究表明,ICRF-193 由于其与拓扑异构酶 IIβ 的强相互作用,是对抗蒽环类药物对心肌细胞毒性最有效的双二氧代哌嗪摘要:右雷佐生 (ICRF-187) 是唯一临床批准的抗蒽环类药物引起的心脏毒性的药物,其心脏保护活性传统上归因于其铁螯合代谢物。然而,最近的实验证据表明,右雷佐生抑制和/或消耗拓扑异构酶 IIβ (TOP2B) 可能具有心脏保护作用。因此,我们评估了一系列右雷佐生类似物,发现它们的心脏保护活性与其与心肌细胞中TOP2B的相互作用密切相关,但与其铁螯合能力无关。 4,4'-(丁烷-2,3-二基)双(哌嗪-2,6-二酮)的立体异构形式证明了非常紧密的结构-活性关系。与其外消旋形式12相比,内消旋衍生物11 (ICRF-193)在计算机模拟中显示出与拓扑异构酶 II 良好的结合模式,比右雷佐生更有效地抑制和耗尽心肌细胞中的 TOP2B,并显示出最高的心脏保护效率。重要的是,观察到的 ICRF-193 心脏保护作用不会干扰蒽环类药物的抗增殖活性。因此,本研究将 ICRF-193 确定为开发有效心脏保护剂的新先导化合物。DOI:10.1021/acs.jmedchem.0c02157
文献信息
-
[EN] ENGINEERED ANTIMICROBIAL AMPHIPHILIC PEPTIDES AND METHODS OF USE<br/>[FR] PEPTIDES AMPHIPHILES ANTIMICROBIENS MODIFIÉS ET PROCÉDÉS D'UTILISATION申请人:PEPTILOGICS INC公开号:WO2018160997A1公开(公告)日:2018-09-07Disclosed herein are novel peptides that can comprise antimicrobial, antiviral, antifungal or antitumor activity when administered to a subject.本文披露了一种新型肽,当向受试者施用时,可以具有抗菌、抗病毒、抗真菌或抗肿瘤活性。
-
MULTINUCLEAR COMPLEX AND CONDENSATION PRODUCT THEREOF申请人:Sugahara Yoshiyuki公开号:US20090036687A1公开(公告)日:2009-02-05A multinuclear complex comprising a plurality of metal atoms and a ligand L coordinating to the metal atoms, and satisfying the following conditions (i), (ii), (iii) and (iv): (i) The ligand L has a monovalent group represented by the following general formula (1) and/or a divalent group represented by the following general formula (2), (ii) The ligand L has at least 5 coordination atoms bonding to the metal atom, (iii) At least one of the coordination atoms bonds to two of the metal atoms, or the minimum number of covalent bonds between any two selected coordination atoms is 1-5, and (iv) The ligand L is soluble in the solvent.
-
A Mechanistic Study on the Reaction of Non‐Heme Diiron(III)‐Peroxido Complexes with Benzoyl Chloride作者:Markus Lerch、Andreas J. Achazi、Doreen Mollenhauer、Jonathan Becker、Siegfried SchindlerDOI:10.1002/ejic.202100711日期:2021.10.21A rapid reaction between a dinuclear iron(III)peroxido complex and benzoyl chloride was observed in methanol. Time-resolved UV-vis spectra at low temperatures showed a clean decay of the peroxido complex with simultaneous formation of an intermediate that we assign as an iron(IV)oxido/benzoato radical species.
-
Preparation and Structure of the Novel Dinuclear Vanadium(III) Complex Bridged by an Alkoxo Group and a Carboxylato Group作者:Kan Kanamori、Kaori Yamamoto、Ken-ichi OkamotoDOI:10.1246/cl.1993.1253日期:1993.7The reaction of 1,3-diamino-2-propanol-N,N,N′,N′-tetraacetate (dpot) with VCl3 followed by the addition of benzoic acid (Hbza) or hydroxybenzoic acid (Hhbza) has yielded the dinuclear vanadium(III) complex, [V2(dpot)(bza or hbza)(H2O)2], bridged by both a deprotonated alkoxo group of dpot and a carboxylato group of bza or hbza.
-
Hemocyanin models: synthesis, structure, and magnetic properties of a binucleating copper(II) system作者:Vickie McKee、Maruta Zvagulis、Jeffrey V. Dagdigian、Marianne G. Patch、Christopher A. ReedDOI:10.1021/ja00329a021日期:1984.8Etude des structures cristallines du complexe acetate ferromagnetique [Cu 2 (L-Et)(OAc)][ClO 4 ] 2 et du complexe azoturo diamagnetique [Cu 2 (L-Et)(N 3 )](BF 4 ) 2 , HL-Et etant le coordinat heptadente N,N,N',N'-tetrakis(ethyl-1 benzimidazolyl-2) hydroxy-2 diamino-1,3 propane
表征谱图
-
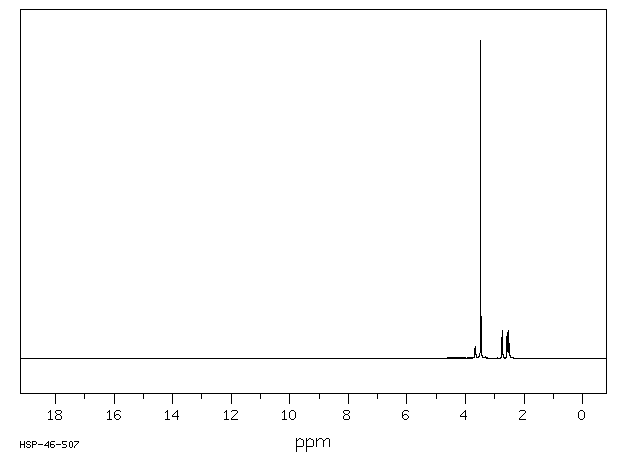
氢谱1HNMR
-
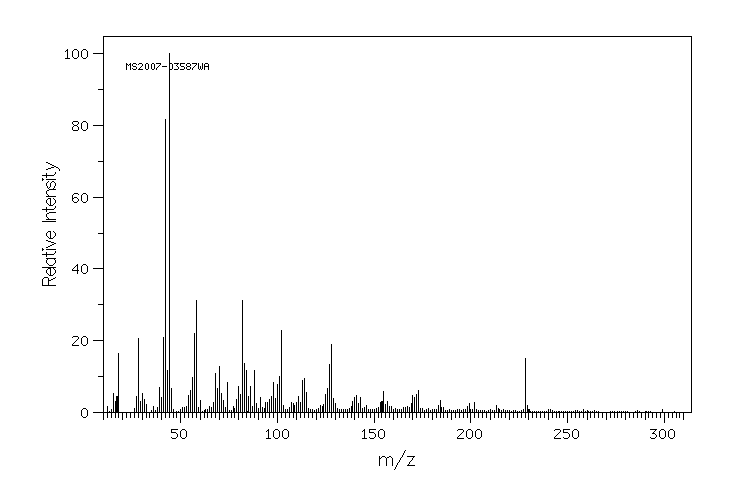
质谱MS
-
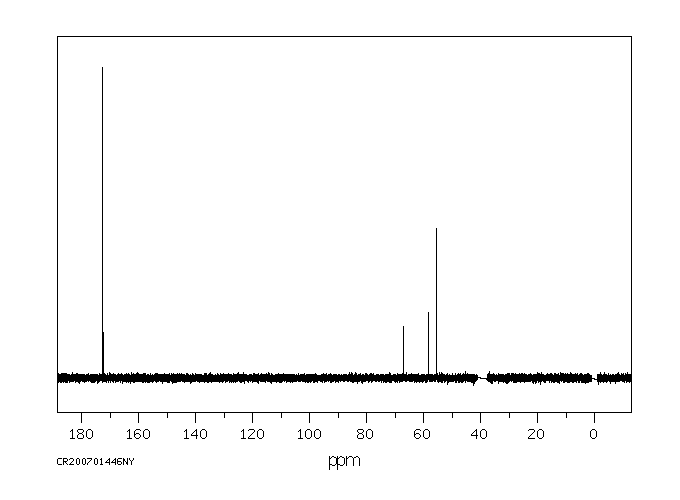
碳谱13CNMR
-
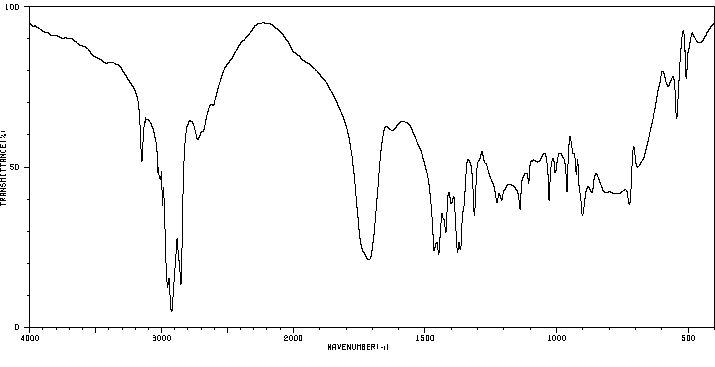
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸