1,7,7-三甲基-2-亚甲基-降冰片烷 | 27538-47-2
中文名称
1,7,7-三甲基-2-亚甲基-降冰片烷
中文别名
——
英文名称
2-methylenebornane
英文别名
2-Methylenbornan;camphor;1,7,7-trimethyl-2-methylidenebicyclo[2.2.1]heptane
CAS
27538-47-2
化学式
C11H18
mdl
——
分子量
150.264
InChiKey
ZASFWGOMAIPHLN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:68-70 °C
-
沸点:167.9±7.0 °C(Predicted)
-
密度:0.88±0.1 g/cm3(Predicted)
-
保留指数:1019.8
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:11
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.82
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2902199090
SDS
上下游信息
反应信息
-
作为反应物:描述:1,7,7-三甲基-2-亚甲基-降冰片烷 、 乙酰丙酮 在 ammonium cerium(IV) nitrate 作用下, 以 甲醇 为溶剂, 以81%的产率得到1-(1',5,7',7'-tetramethylspiro[3H-furan-2,2'-bicyclo[2.2.1]heptane]-4-yl)ethanone参考文献:名称:Oxidative [3+2] Cycloaddition Reactions of 1,3-Dicarbonyl Compounds to Exocyclic Alkenes: The Regiospecific Synthesis of Spirodihydrofurans摘要:使用硝酸铈(IV)铵介导的1,3-二羰基化合物与环外烯烃的氧化[3+2]环加成反应实现了多种螺二氢呋喃的区域特异性合成。DOI:10.1055/s-2002-25334
-
作为产物:描述:参考文献:名称:Reduction of [(phenylthio)methyl]carbinyl benzoate esters to alkenes with titanium metal摘要:DOI:10.1021/jo00333a030
文献信息
-
Dichloromethane Activation. Direct Methylenation of Ketones and Aldehydes with CH<sub>2</sub>Cl<sub>2</sub> Promoted by Mg/TiCl<sub>4</sub>/THF作者:Tu-Hsin Yan、Chia-Chung Tsai、Ching-Ting Chien、Chia-Ching Cho、Pei-Chen HuangDOI:10.1021/ol0478887日期:2004.12.1[reaction: see text] This Mg-TiCl4-promoted CH2-transfer reaction of CH2Cl2 represents an extremely simple, practical, and efficient methylenation of a variety of ketones and aldehydes, especially in enolizable or sterically hindered ketones such as 2,2-dimethylcyclohexanone, camphor, and fenchone.[反应:查看文本]这种由Mg-TiCl4促进的CH2Cl2的CH2转移反应代表了各种酮和醛的极其简单,实用和有效的亚甲基化,特别是在可烯醇化或空间受阻的酮(如2,2-二甲基环己酮)中,樟脑和fenchone。
-
Lewis acid catalysis of the ene addition of chloral and bromal to olefins; stereochemical and mechanistic studies作者:Jill P. Benner、G. Bryon Gill、Stephen J. Parrott、Brian Wallace、Michael J. BegleyDOI:10.1039/p19840000315日期:——the additions to olefins of moderate reactivity) indicating the participation of Friedel-Crafts type dipolar intermediates. The ene adducts themselves could be formed via dipolar intermediates or in competing ‘concerted’ reactions; the stepwise mechanism must operate in some reactions because of the observation of Wagner-Meerwein rearrangements. Olefin reactivity over the series, measured by the competitive路易斯酸催化的将氯醛和溴的烯加到烯烃上是完全区域特异性的,具有中等区域选择性的,并且常常是高度立体选择性的。除(-)-β-pine烯外,非对映选择性是路易斯酸的作用,在TiCl 4中基本上观察到定量不对称诱导。通过假设活性的亲核体具有诸如(1)之类的transoid结构,协调一致或快速的逐步机制起作用,以及产物形成主要通过烯烃与(1)的受最小阻碍的相遇配合物发生,可以令人满意地解释立体化学现象。但是,对于2-甲基丁-2-烯,有一些证据证明了另外的立体电子贡献,即顺式-影响'。X射线结构数据支持立体化学分配。形成酮,氢卤化的烯烃加合物或重排产物(主要是向中等反应性的烯烃添加),表明Friedel-Crafts型偶极中间体的参与。烯加成物本身可以通过偶极中间体或竞争性“一致”反应形成。由于观察到Wagner-Meerwein重排,所以步进机制必须在某些反应中起作用。烯烃的反应性在系列,通过竞争性技术来测量,向氯醛-的AlCl
-
Oxone-mediated Methoxy Thiocyanation of 1,1-Disubstituted Olefins in Methanol作者:Guaili Wu、Wentao Wu、Rui Li、Yinglin Shen、Longmin WuDOI:10.1246/cl.2007.188日期:2007.1Reactions of 1,1-disubstituted olefins with oxone and ammonium thiocyanate in methanol gave the corresponding 1-methoxy-2-thiocyano adducts in various yields.
-
Wittig-type Reaction of Dimetallated Carbodianion Species as Produced by Zinc Reduction of<i>gem</i>-Polyhalogen Compounds in the Presence of Lewis Acids作者:Kazuhiko Takai、Yuji Hotta、Koichiro Oshima、Hitosi NozakiDOI:10.1246/bcsj.53.1698日期:1980.6Treatment of R1COR2 with a suspension prepared from diiodomethane, trimethylaluminum and excess zinc in tetrahydrofuran at room temperature affords olefins R1R2C=CH2 in fair (R1,R2=alkyl) to good (R1=alkyl, R2=H) yields. The ketone methylenation is better carried with another system consisting of CH2Br2–Zn–TiCl4. Ketones and aldehydes are transformed into α-chloro α,β-unsaturated esters or α,β-unsaturated
-
Reactions of Cyclic Diazoamides:Convenient Synthesis of Dispirocyclic Cyclopropane Systems作者:Sengodagounder Muthusamy、Chidambaram GunanathanDOI:10.1055/s-2003-40996日期:——Rhodium(II) acetate catalyzed intermolecular cyclopropanationreactions of cyclic diazoamides with exocyclic olefins are describedto furnish an assortment of stable strained dispirocyclic cyclopropanesystems in good yields under mild experimental conditions.
表征谱图
-
氢谱1HNMR
-
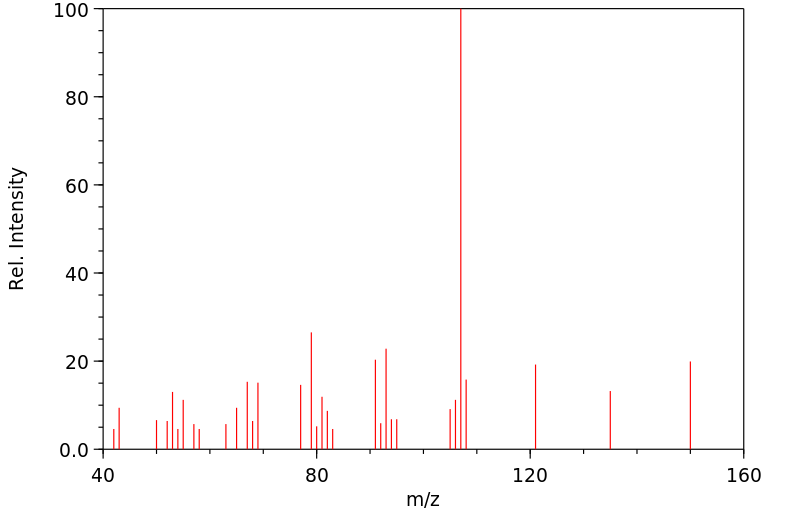
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β,6α,8α,10α,13α)-6-羟基-15-氧代黄-9(11),16-二烯-18-油酸
(3S,3aR,8aR)-3,8a-二羟基-5-异丙基-3,8-二甲基-2,3,3a,4,5,8a-六氢-1H-天青-6-酮
(2Z)-2-(羟甲基)丁-2-烯酸乙酯
(2S,4aR,6aR,7R,9S,10aS,10bR)-甲基9-(苯甲酰氧基)-2-(呋喃-3-基)-十二烷基-6a,10b-二甲基-4,10-dioxo-1H-苯并[f]异亚甲基-7-羧酸盐
(1aR,4E,7aS,8R,10aS,10bS)-8-[((二甲基氨基)甲基]-2,3,6,7,7a,8,10a,10b-八氢-1a,5-二甲基-氧杂壬酸[9,10]环癸[1,2-b]呋喃-9(1aH)-酮
(+)顺式,反式-脱落酸-d6
龙舌兰皂苷乙酯
龙脑香醇酮
龙脑烯醛
龙脑7-O-[Β-D-呋喃芹菜糖基-(1→6)]-Β-D-吡喃葡萄糖苷
龙牙楤木皂甙VII
龙吉甙元
齿孔醇
齐墩果醛
齐墩果酸苄酯
齐墩果酸甲酯
齐墩果酸溴乙酯
齐墩果酸二甲胺基乙酯
齐墩果酸乙酯
齐墩果酸3-O-alpha-L-吡喃鼠李糖基(1-3)-beta-D-吡喃木糖基(1-3)-alpha-L-吡喃鼠李糖基(1-2)-alpha-L-阿拉伯糖吡喃糖苷
齐墩果酸 beta-D-葡萄糖酯
齐墩果酸 beta-D-吡喃葡萄糖基酯
齐墩果酸 3-乙酸酯
齐墩果酸 3-O-beta-D-葡吡喃糖基 (1→2)-alpha-L-吡喃阿拉伯糖苷
齐墩果酸
齐墩果-12-烯-3b,6b-二醇
齐墩果-12-烯-3,24-二醇
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,11-二酮
齐墩果-12-烯-2α,3β,28-三醇
齐墩果-12-烯-29-酸,3,22-二羟基-11-羰基-,g-内酯,(3b,20b,22b)-
齐墩果-12-烯-28-酸,3-[(6-脱氧-4-O-b-D-吡喃木糖基-a-L-吡喃鼠李糖基)氧代]-,(3b)-(9CI)
齐墩果-12-烯-28-酸,3,7-二羰基-(9CI)
齐墩果-12-烯-28-酸,3,21,29-三羟基-,g-内酯,(3b,20b,21b)-(9CI)
鼠特灵
鼠尾草酸醌
鼠尾草酸
鼠尾草酚酮
鼠尾草苦内脂
黑蚁素
黑蔓醇酯B
黑蔓醇酯A
黑蔓酮酯D
黑海常春藤皂苷A1
黑檀醇
黑果茜草萜 B
黑五味子酸
黏黴酮
黏帚霉酸