2,3-二溴丁酸 | 600-30-6
物质功能分类
中文名称
2,3-二溴丁酸
中文别名
——
英文名称
2,3-dibromobutanoic acid
英文别名
2,3-dibromo-butyric acid;2,3-Dibrom-buttersaeure;α.β-Dibrombuttersaeure;2,3-Dibromobutyric acid
CAS
600-30-6
化学式
C4H6Br2O2
mdl
——
分子量
245.898
InChiKey
HESQKTULJLBDRF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:86-90 °C
-
沸点:100-110°C 2mm
-
密度:1.9671 (rough estimate)
-
闪点:100-110°C/2mm
-
稳定性/保质期:
性质与稳定性:在常温常压下,该物质不会分解产生其他产物。
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:8
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险等级:8
-
危险品标志:C
-
危险类别码:R34
-
危险品运输编号:3261
-
海关编码:2915900090
-
包装等级:II
-
危险类别:8
-
安全说明:S26,S28A
-
储存条件:贮存: 将密封的药器储存在密封的主要容器中,并放置于阴凉、干燥处。
SDS
| Name: | 2 3-Dibromobutyric Acid 98% Material Safety Data Sheet |
| Synonym: | None known |
| CAS: | 600-30-6 |
Synonym:None known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 600-30-6 | 2,3-Dibromobutyric Acid | 98 | 209-992-1 |
Risk Phrases: 34
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Causes burns.Corrosive.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
Causes eye burns. May cause chemical conjunctivitis and corneal damage.
Skin:
Causes skin burns. May cause skin rash (in milder cases), and cold and clammy skin with cyanosis or pale color.
Ingestion:
May cause severe and permanent damage to the digestive tract. Causes gastrointestinal tract burns. May cause perforation of the digestive tract. The toxicological properties of this substance have not been fully investigated. May cause systemic effects.
Inhalation:
May cause severe irritation of the respiratory tract with sore throat, coughing, shortness of breath and delayed lung edema. Causes chemical burns to the respiratory tract. The toxicological properties of this substance have not been fully investigated.
Aspiration may lead to pulmonary edema. May cause systemic effects.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Get medical aid. Do NOT allow victim to rub eyes or keep eyes closed. Extensive irrigation with water is required (at least 30 minutes).
Skin:
Get medical aid immediately. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse. Destroy contaminated shoes.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Use water spray to keep fire-exposed containers cool. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Non-combustible, substance itself does not burn but may decompose upon heating to produce irritating, corrosive and/or toxic fumes.
Extinguishing Media:
Do NOT get water inside containers. For small fires, use dry chemical, carbon dioxide, or water spray. For large fires, use dry chemical, carbon dioxide, alcohol-resistant foam, or water spray.
Cool containers with flooding quantities of water until well after fire is out.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation.
Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Do not breathe dust, vapor, mist, or gas. Keep container tightly closed. Avoid ingestion and inhalation. Discard contaminated shoes.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Corrosives area.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 600-30-6: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: white
Odor: none reported
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 86.00 - 88.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C4H6Br2O2
Molecular Weight: 245.90
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, hydrogen bromide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 600-30-6 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2,3-Dibromobutyric Acid - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: CORROSIVE SOLID, ACIDIC, ORGANIC, N.O.S.*
Hazard Class: 8
UN Number: 3261
Packing Group: III
IMO
Shipping Name: CORROSIVE SOLID, ACIDIC, ORGANIC, N.O.S.
Hazard Class: 8
UN Number: 3261
Packing Group: III
RID/ADR
Shipping Name: CORROSIVE SOLID, ACIDIC, ORGANIC, N.O.S.
Hazard Class: 8
UN Number: 3261
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: C
Risk Phrases:
R 34 Causes burns.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 600-30-6: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 600-30-6 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 600-30-6 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:参考文献:名称:邻位二溴化物的催化脱溴摘要:使用硼氢化钠和催化量的双(2-噻吩基)二碲化物将许多邻位二溴化物脱溴。DOI:10.1016/s0040-4039(00)87681-9
-
作为产物:参考文献:名称:Matveeva; Erin; Kurz, Russian Journal of Organic Chemistry, 1997, vol. 33, # 8, p. 1065 - 1067摘要:DOI:
文献信息
-
Carbene-catalyzed LUMO activation of alkyne esters for access to functional pyridines作者:Chengli Mou、Jichang Wu、Zhijian Huang、Jun Sun、Zhichao Jin、Yonggui Robin ChiDOI:10.1039/c7cc08039e日期:——A carbene-catalyzed LUMO activation of α,β-unsaturated alkyne esters is reported. This catalytic process allows for effective reactions of alkyne esters with enamides to synthesize functional pyridines via simple protocols. A previously unexplored unsaturated alkyne acyl azolium intermediate is involved in the key step of the reaction.
-
α-Bromo-β,γ-unsaturated ketenes for the synthesis of α-benzylamino-β,γ-unsaturated acids作者:Giuliana Cardillo、Serena Fabbroni、Luca Gentilucci、Rossana Perciaccante、Alessandra TolomelliDOI:10.1016/j.tetasy.2003.12.029日期:2004.2The synthesis of α-benzylamino-β,γ-unsaturated acids has been developed starting from α-bromo-α,β-unsaturated chlorides. Via treatment of the acyl chlorides with (R)-pantolactone in the presence of TEA, the in situ formation of the deconjugated ketenes and their direct transformation into chiral esters was performed. The substitution of bromine with benzylamine, followed by acid hydrolysis, allowed
-
Selective debromination of activated vicinal dibromides by copper promoted by copper(II)
-
The Action of Methylmagnesium Iodide on Methyl α-Phenylcinnamate and a Synthesis of 1,1-Dimethyl-2-phenylindene作者:C. F. Koelsch、Paul R. JohnsonDOI:10.1021/ja01244a020日期:1943.4
-
461. Alkenylation employing lithium alkenyls. Part V. The formation and some reactions of cis-propenyl-lithium作者:E. A. Braude、J. A. ColesDOI:10.1039/jr9510002078日期:——
表征谱图
-
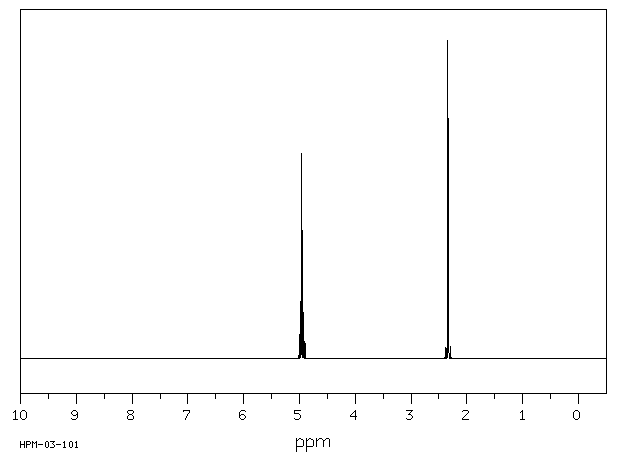
氢谱1HNMR
-
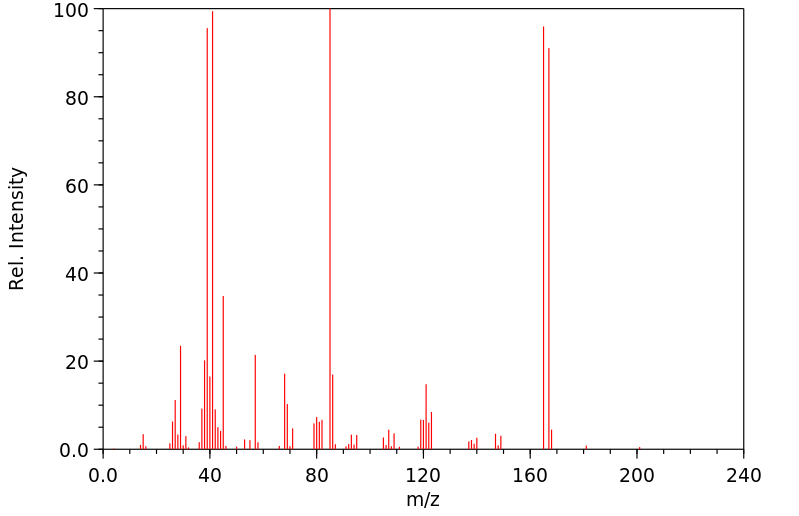
质谱MS
-
碳谱13CNMR
-
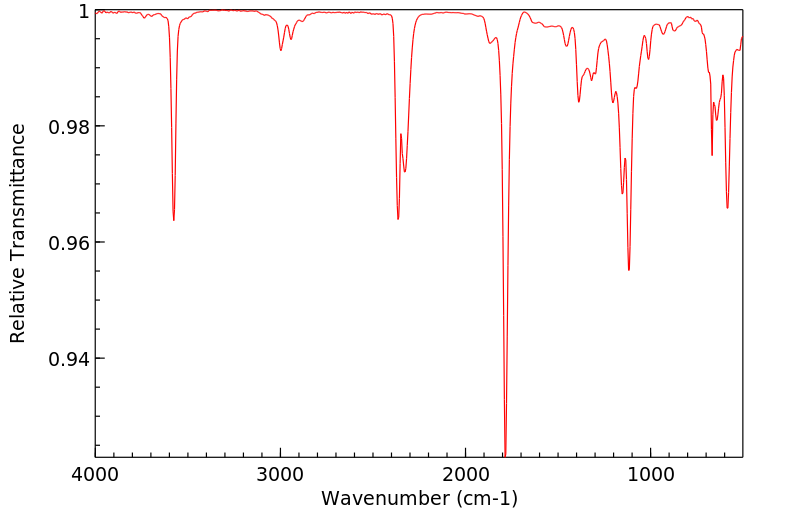
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯