2-乙基蒽 | 52251-71-5
中文名称
2-乙基蒽
中文别名
——
英文名称
2-ethylanthracene
英文别名
2-Aethyl-anthracen
CAS
52251-71-5
化学式
C16H14
mdl
MFCD00003584
分子量
206.287
InChiKey
ZXAGXLDEMUNQSH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:152-153 °C(lit.)
-
沸点:364.3±9.0 °C(Predicted)
-
密度:1.082±0.06 g/cm3(Predicted)
-
溶解度:1.30e-07 M
-
蒸汽压力:5.24e-06 mmHg
-
保留指数:337.28
-
稳定性/保质期:
- 遵照规定使用和储存则不会分解。
- 存在于烟气中。
计算性质
-
辛醇/水分配系数(LogP):5
-
重原子数:16
-
可旋转键数:1
-
环数:3.0
-
sp3杂化的碳原子比例:0.125
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2902909090
-
包装等级:III
-
危险类别:9
-
危险性防范说明:P273,P280,P305+P351+P338,P501
-
危险品运输编号:3077
-
危险性描述:H318,H410
-
储存条件:保持贮藏器密封,并将其放入一个紧密封装的容器中。建议储存在阴凉、干燥的地方。
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product identifiers
Product name : 2-Ethylanthracene
CAS-No. : 52251-71-5
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008 [EU-GHS/CLP]
Serious eye damage (Category 1)
Acute aquatic toxicity (Category 1)
Chronic aquatic toxicity (Category 1)
Classification according to EU Directives 67/548/EEC or 1999/45/EC
Risk of serious damage to eyes. Very toxic to aquatic organisms, may cause long-term adverse effects in
the aquatic environment.
Label elements
Labelling according Regulation (EC) No 1272/2008 [CLP]
Pictogram
Signal word Danger
Hazard statement(s)
H318 Causes serious eye damage.
H410 Very toxic to aquatic life with long lasting effects.
Precautionary statement(s)
P273 Avoid release to the environment.
P280 Wear protective gloves/ eye protection/ face protection.
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove
contact lenses, if present and easy to do. Continue rinsing.
P501 Dispose of contents/ container to an approved waste disposal plant.
Supplemental Hazard none
Statements
According to European Directive 67/548/EEC as amended.
Hazard symbol(s)
R-phrase(s)
R41 Risk of serious damage to eyes.
R50/53 Very toxic to aquatic organisms, may cause long-term adverse effects in
the aquatic environment.
S-phrase(s)
S26 In case of contact with eyes, rinse immediately with plenty of water and
seek medical advice.
S39 Wear eye/face protection.
S60 This material and its container must be disposed of as hazardous waste.
S61 Avoid release to the environment. Refer to special instructions/ Safety
data sheets.
Other hazards - none
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Formula : C16H14
Molecular Weight : 206,28 g/mol
Component Concentration
2-Ethylanthracene
CAS-No. 52251-71-5 -
EC-No. 257-788-6
Section 4. FIRST AID MEASURES
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Consult a physician.
In case of eye contact
Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.
Most important symptoms and effects, both acute and delayed
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Indication of any immediate medical attention and special treatment needed
no data available
Section 5. FIREFIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid dust formation. Avoid breathing vapors, mist or gas. Ensure
adequate ventilation. Evacuate personnel to safe areas. Avoid breathing dust.
Environmental precautions
Prevent further leakage or spillage if safe to do so. Do not let product enter drains. Discharge into the
environment must be avoided.
Methods and materials for containment and cleaning up
Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Avoid formation of dust and aerosols.
Provide appropriate exhaust ventilation at places where dust is formed.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Specific end uses
no data available
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and
at the end of workday.
Personal protective equipment
Eye/face protection
Face shield and safety glasses Use equipment for eye protection tested and approved under
appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
Complete suit protecting against chemicals, The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Where risk assessment shows air-purifying respirators are appropriate use a full-face particle
respirator type N100 (US) or type P3 (EN 143) respirator cartridges as a backup to engineering
controls. If the respirator is the sole means of protection, use a full-face supplied air respirator. Use
respirators and components tested and approved under appropriate government standards such
as NIOSH (US) or CEN (EU).
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
a) Appearance Form: solid
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing Melting point/range: 152 - 153 °C - lit.
point
f) Initial boiling point and no data available
boiling range
g) Flash point no data available
h) Evaporation rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility no data available
o) Partition coefficient: n- log Pow: 5,369
octanol/water
p) Autoignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
Section 10. STABILITY AND REACTIVITY
Reactivity
no data available
Chemical stability
no data available
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
Section 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Potential health effects
Inhalation May be harmful if inhaled. May cause respiratory tract irritation.
Ingestion May be harmful if swallowed.
Skin May be harmful if absorbed through skin. May cause skin irritation.
Eyes Causes eye burns.
Signs and Symptoms of Exposure
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Additional Information
RTECS: Not available
Section 12. ECOLOGICAL INFORMATION
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
no data available
Other adverse effects
Very toxic to aquatic life with long lasting effects.
Section 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company. Dissolve or mix the material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
UN number
ADR/RID: 3077 IMDG: 3077 IATA: 3077
UN proper shipping name
ADR/RID: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S. (2-Ethylanthracene)
IMDG: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S. (2-Ethylanthracene)
IATA: Environmentally hazardous substance, solid, n.o.s. (2-Ethylanthracene)
Transport hazard class(es)
ADR/RID: 9 IMDG: 9 IATA: 9
Packaging group
ADR/RID: III IMDG: III IATA: III
Environmental hazards
ADR/RID: yes IMDG Marine pollutant: yes IATA: yes
Special precautions for user
Further information
EHS-Mark required (ADR 2.2.9.1.10, IMDG code 2.10.3) for single packagings and combination
packagings containing inner packagings with Dangerous Goods > 5L for liquids or > 5kg for solids.
SECTION 15 - REGULATORY INFORMATION
N/A
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:参考文献:名称:无溶剂条件下在Cu / SBA-15催化剂上烷基芳烃的选择性苄基氧化摘要:为了在无溶剂条件下选择性地将烷基芳族化合物苄基氧化为相应的酮,通过浸渍SBA-制备了廉价,简单且通用的Cu / SBA-15催化剂体系,其铜载量为5、10、15和20%。 15支持。在Cu / SBA-15催化剂中,10%Cu / SBA-15表现出优异的活性和选择性。DOI:10.1016/j.catcom.2013.04.023
-
作为产物:描述:参考文献:名称:铝粉在水性介质中InCl 3催化新的蒽酮和蒽醌还原摘要:在不同条件下研究了InCl 3催化的蒽酮和蒽醌的还原。描述了一种在温和条件下在水性介质中合成蒽的新方法。DOI:10.1016/j.tet.2007.03.113
文献信息
-
MANGANESE (III) CATALYZED C--H AMINATIONS申请人:The Board of Trustees of the University of Illinois公开号:US20190106448A1公开(公告)日:2019-04-11Reactions that directly install nitrogen into C—H bonds of complex molecules are significant because of their potential to change the chemical and biological properties of a given compound. Selective intramolecular C—H amination reactions that achieve high levels of reactivity, while maintaining excellent site-selectivity and functional-group tolerance is a challenging problem. Herein is reported a manganese perchlorophthalocyanine catalyst [Mn III (ClPc)] for intermolecular benzylic C—H amination of bioactive molecules and natural products that proceeds with unprecedented levels of reactivity and site-selectivity. In the presence of Brønsted or Lewis acid, the [Mn III (ClPc)]-catalyzed C—H amination demonstrates unique tolerance for tertiary amine, pyridine and benzimidazole functionalities. Mechanistic studies indicate that C—H amination proceeds through an electrophilic metallonitrene intermediate via a stepwise pathway where C—H cleavage is the rate-determining step of the reaction. Collectively these mechanistic features contrast previous base-metal catalyzed C—H aminations.直接将氮原子安装到复杂分子中的C-H键的反应具有重要意义,因为它们有可能改变给定化合物的化学和生物性质。实现高反应性,同时保持优秀的位点选择性和官能团耐受性的选择性分子内C-H胺化反应是一个具有挑战性的问题。本文报道了一种锰过氯酞菁催化剂[MnIII(ClPc)],用于分子间苄基C-H胺化反应,该反应在生物活性分子和天然产物中以前所未有的反应性和位点选择性进行。在Brønsted酸或Lewis酸的存在下,[MnIII(ClPc)]催化的C-H胺化展示了对叔胺、吡啶和苯并咪唑官能团的独特耐受性。机理研究表明,C-H胺化通过亲电金属亚硝烯中间体经过分步途径进行,其中C-H裂解是反应的速率决定步骤。总的来说,这些机理特征与之前基于贱金属催化的C-H胺化反应形成了对比。
-
An efficient and selective benzylic oxidation of tetralin to 1-tetralone on Cu(II) immobilized γ-Fe 2 O 3 @SBA-15 magnetic nanocatalyst in green water medium without base or additives作者:Chinna Krishna Prasad Neeli、Hari Prasad Reddy Kannapu、Venkat Narayana Kalevaru、Seetha Rama Rao Kamaraju、David Raju BurriDOI:10.1016/j.mcat.2018.04.030日期:2018.7A novel Cu(II)/γ-Fe2O3@SBA-15 magnetic nanocatalyst has been synthesized aiming at possessing high surface area, magnetic property, highly dispersed and stabilized Cu(II) complex using SBA-15 by sequential magnetization and Cu(II) immobilization with Fe(acac)3 and Cu(OAc)2 precursors. The nanocatalyst was well authenticated by different techniques like BET surface area, X-ray diffraction, X-ray photoelectron一种新颖的Cu(II)/γ -铁2 ö 3 @ SBA-15的磁性纳米催化剂已经合成瞄准具有高表面积,磁特性,高度分散和稳定的Cu(II)配合物使用SBA-15通过顺序磁化和Cu (II)用Fe(acac)3和Cu(OAc)2前体固定化。纳米催化剂通过不同的技术得到了很好的验证,例如BET表面积,X射线衍射,X射线光电子能谱,FT-IR,振动样品磁力计和热重分析。铜(II)/γ -铁2 ö 3发现TBHP在绿水介质中温和的反应条件下,即使在没有任何碱或添加剂的情况下,也可使用@ SBA-15将四氢化萘苄基氧化为1-四氢萘酮。通过热过滤测试确认了催化剂的非均质性,并进一步重复使用了至少4次,而没有明显的催化活性损失。易于分离催化剂,可重复使用的容量,烷基芳烃的广泛应用以及通过消除有害有机溶剂而在水中作为绿色介质进行的重要反应操作,都是该催化剂值得称赞的优点。
-
Nitrous Oxide Oxidation Catalyzed by Ruthenium Porphyrin Complex作者:Hirotaka Tanaka、Kentaro Hashimoto、Kyosuke Suzuki、Yasunori Kitaichi、Mitsuo Sato、Taketo Ikeno、Tohru YamadaDOI:10.1246/bcsj.77.1905日期:2004.10Dinitrogen oxide was employed as a clean oxidant for various oxidations in the presence of a catalytic amount of dioxoruthenium tetramesitylporphyrin complex (Ru(tmo)(O) 2 ). A variety of olefins, secondary alcohols, and benzyl alcohols were smoothly oxidized to the corresponding epoxides, ketones, and aldehydes in high yields. In the oxidation of 9,10-dihydroanthracene derivatives, the competitive
-
Anthracene derivatives申请人:Burroughs Wellcome Co.公开号:US04719049A1公开(公告)日:1988-01-12The present invention relates to compounds of formula (I) ArCH.sub.2 R.sup.1 (I) or a monomethyl or a monoethyl ether thereof (the compound of formula (I) including these ethers may contain no more than 30 carbon atoms in total); ethers, esters thereof; acid addition salts thereof; wherein Ar is an anthracene or substituted anthracene ring system; R.sup.1 contains not more than eight carbon carbon atoms and is a group ##STR1## wherein m is 0 or 1; R.sup.5 is hydrogen; R.sup.6 and R.sup.7 are the same or different and each is hydrogen or C.sub.1-3 alkyl optionally substituted by hydroxy; R.sup.8 and R.sup.9 are the same or different and each is hydrogen or C.sub.1-3 alkyl; --C--C-- is a five- or six-membered saturated carbocyclic ring; R.sup.10 is hydrogen, methyl or hydroxymethyl; R.sup.11, R.sup.12 and R.sup.13 are the same or different and each is hydrogen or methyl; R.sup.14 is hydrogen, methyl, hydroxy, or hydroxymethyl.本发明涉及通式(I)ArCH.sub.2 R.sup.1 (I)的化合物或其单甲基或单乙基醚(通式(I)的化合物包括这些醚,总共含有不超过30个碳原子);其醚、酯;其酸加成盐;其中Ar为蒽或取代蒽环系;R.sup.1含有不超过八个碳碳原子,为如下基团##STR1##其中m为0或1;R.sup.5为氢;R.sup.6和R.sup.7相同或不同,各自为氢或C.sub.1-3烷基,可选择性地被羟基取代;R.sup.8和R.sup.9相同或不同,各自为氢或C.sub.1-3烷基;--C--C--为五元或六元饱和碳环;R.sup.10为氢、甲基或羟甲基;R.sup.11、R.sup.12和R.sup.13相同或不同,各自为氢或甲基;R.sup.14为氢、甲基、羟基或羟甲基。
-
Nickel-Catalyzed Cross-Coupling of Organolithium Reagents with (Hetero)Aryl Electrophiles作者:Dorus Heijnen、Jean-Baptiste Gualtierotti、Valentín Hornillos、Ben L. FeringaDOI:10.1002/chem.201505106日期:2016.3.14Nickel‐catalyzed selective cross‐coupling of aromatic electrophiles (bromides, chlorides, fluorides and methyl ethers) with organolithium reagents is presented. The use of a commercially available nickel N‐heterocyclic carbene (NHC) complex allows the reaction with a variety of (hetero)aryllithium compounds, including those prepared via metal‐halogen exchange or direct metallation, whereas a commercially
表征谱图
-
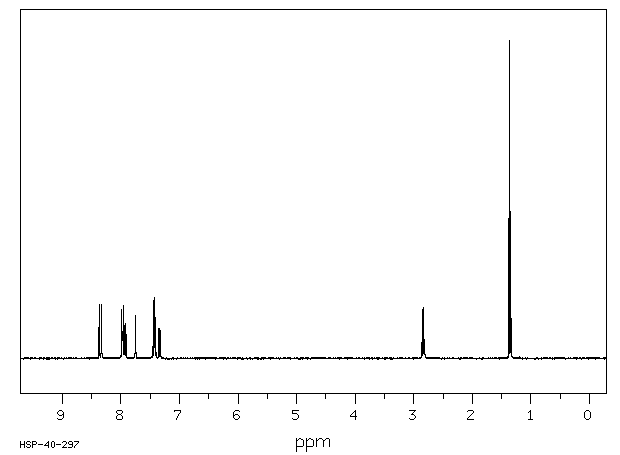
氢谱1HNMR
-
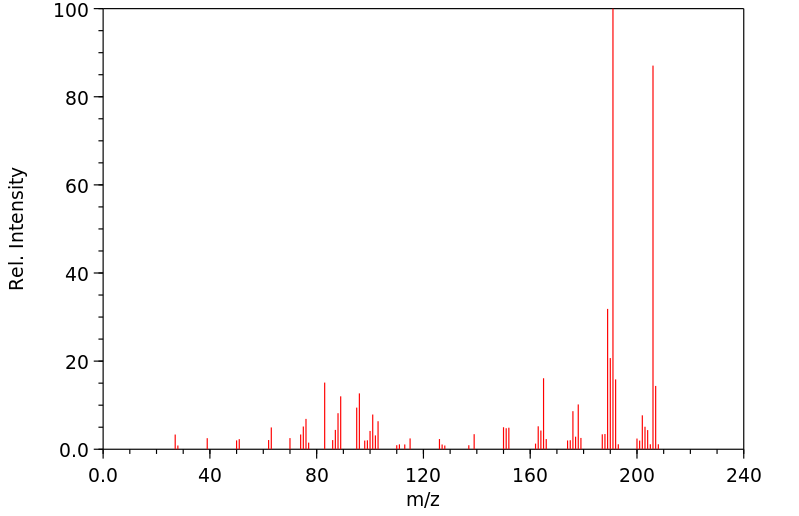
质谱MS
-
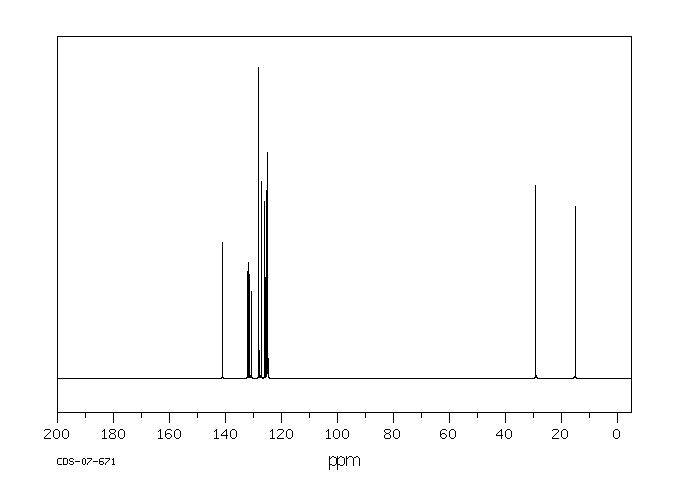
碳谱13CNMR
-
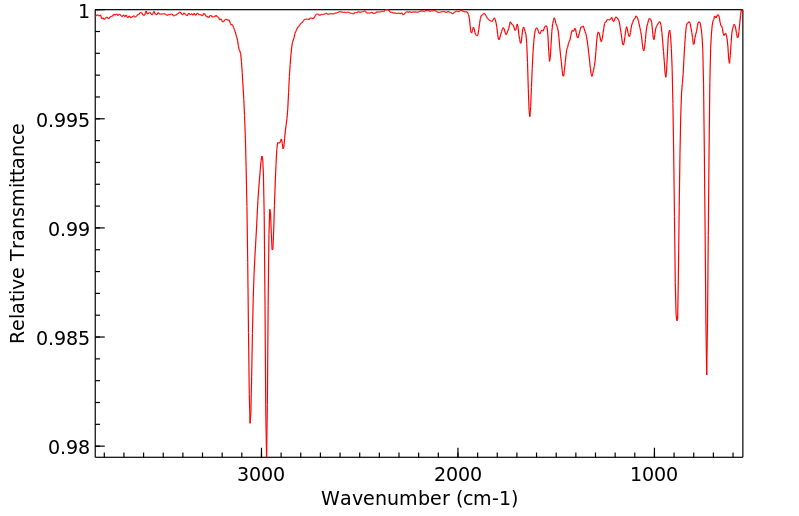
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
齐斯托醌
黄决明素
马普替林相关物质D
马普替林杂质E(N-甲基马普替林)
马普替林杂质D
马普替林D3
马普替林
颜料黄199
颜料黄147
颜料黄123
颜料黄108
颜料红89
颜料红85
颜料红251
颜料红177
颜料紫27
顺式-1-(9-蒽基)-2-硝基乙烯
阿美蒽醌
阳离子蓝FGL
阳离子蓝3RL
长蠕孢素
镁蒽四氢呋喃络合物
镁蒽
锈色洋地黄醌醇
锂钠2-[[4-[[3-[(4-氨基-9,10-二氧代-3-磺基-1-蒽基)氨基]-2,2-二甲基-丙基]氨基]-6-氯-1,3,5-三嗪-2-基]氨基]苯-1,4-二磺酸酯
锂胭脂红
链蠕孢素
铷离子载体I
铝洋红
铂(2+)二氯化1-({2-[(2-氨基乙基)氨基]乙基}氨基)蒽-9,10-二酮(1:1)
钾6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠alpha-(丙烯酰氨基)-[4-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]苯氧基]甲苯磺酸盐
钠[[3-[[4-(环己基氨基)-9,10-二氢-9,10-二氧代-1-蒽基]氨基]-1-氧代丙基]氨基]苯磺酸盐
钠[3-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]丁基]苯磺酸盐
钠6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠4-({4-[乙酰基(乙基)氨基]苯基}氨基)-1-氨基-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠2-[(4-氨基-9,10-二氧代-3-磺基-9,10-二氢-1-蒽基)氨基]-4-{[2-(磺基氧基)乙基]磺酰基}苯甲酸酯
钠1-氨基-9,10-二氢-4-[[4-(1,1-二甲基乙基)-2-甲基苯基]氨基]-9,10-二氧代蒽-2-磺酸盐
钠1-氨基-4-[(3-{[(4-甲基苯基)磺酰基]氨基}苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-[(3,4-二甲基苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-(1,3-苯并噻唑-2-基硫基)-9,10-二氧代蒽-2-磺酸盐
醌茜隐色体
醌茜素
酸性蓝P-RLS
酸性蓝41
酸性蓝27
酸性蓝127:1
酸性紫48
酸性紫43
酸性兰62