2-氨基-2-脱氧-D-半乳糖盐酸盐 | 1772-03-8
中文名称
2-氨基-2-脱氧-D-半乳糖盐酸盐
中文别名
软骨糖胺盐酸盐;D-半乳糖胺盐酸盐;D-半;D-氨基半乳糖盐酸盐;D-(+)-氨基半乳糖盐酸盐;D-(+)-半乳糖胺盐酸盐;2-氨基-2-脱氧-D-半乳糖盐酸盐(D-半乳糖胺盐酸盐);2-氨基-2-脱氧-D半乳糖盐酸盐;2-氨基-2-脱氧D-半乳糖盐酸盐;2-氨基-2-脱氧-D-水解乳糖盐酸盐;D-(+)-氨基葡萄糖盐酸盐
英文名称
2-amino-2-deoxy-D-galactose hydrochloride
英文别名
D-galactosamine hydrochloride;galactosamine hydrochloride;(2R,3R,4R,5R)-2-amino-3,4,5,6-tetrahydroxyhexanal hydrochloride;Galaktosamin-hydrochlorid;(2R,3R,4R,5R)-2-amino-3,4,5,6-tetrahydroxyhexanal;hydrochloride
CAS
1772-03-8
化学式
C6H13NO5*ClH
mdl
——
分子量
215.634
InChiKey
CBOJBBMQJBVCMW-NQZVPSPJSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:182-185 °C (dec.)(lit.)
-
比旋光度:96 º (589nm c=1,H2O,24 hrs)
-
密度:1.3965 (rough estimate)
-
溶解度:H2O:50 mg/mL,澄清,无色
-
稳定性/保质期:
常压稳定,对湿气敏感,并可与氧化物发生反应。
计算性质
-
辛醇/水分配系数(LogP):-2.99
-
重原子数:13
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:0.83
-
拓扑面积:124
-
氢给体数:6
-
氢受体数:6
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S24/25
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2922399090
-
危险品运输编号:NONH for all modes of transport
-
RTECS号:LW5500000
-
危险性防范说明:P261,P280,P305+P351+P338
-
危险性描述:H302,H315,H319,H332,H335
-
储存条件:在2°C至-8°C的条件下密封保存,存放在阴凉干燥处。请避免接触潮湿和水源,并远离氧化剂。
SDS
| Name: | d-(+)-Galactosamine Hydrochloride 99% Material Safety Data Sheet |
| Synonym: | Chondrosamine hydrochloride; 2-Amino-2-deoxy-D-galactose hydrochlorid |
| CAS: | 1772-03-8 |
Synonym:Chondrosamine hydrochloride; 2-Amino-2-deoxy-D-galactose hydrochlorid
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1772-03-8 | D-(+)-Galactosamine hydrochloride | 99 | 217-198-1 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract.
Inhalation:
May cause respiratory tract irritation.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Do not induce vomiting. If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or alcohol-resistant foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up, then place into a suitable container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation.
Minimize dust generation and accumulation. Avoid contact with skin and eyes. Avoid ingestion and inhalation.
Storage:
Store in a cool, dry place. Keep container closed when not in use.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1772-03-8: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: white
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 178 - 190 deg C (dec.)
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: > 190 deg C
Solubility in water: soluble in water
Specific Gravity/Density:
Molecular Formula: C6H13NO5.HCl
Molecular Weight: 215.64
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, chloride fumes.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1772-03-8: LW5500000 LD50/LC50:
Not available.
Carcinogenicity:
D-(+)-Galactosamine hydrochloride - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
WGK (Water Danger/Protection)
CAS# 1772-03-8: No information available.
Canada
CAS# 1772-03-8 is listed on Canada's NDSL List.
CAS# 1772-03-8 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1772-03-8 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
用途
2-氨基-2-脱氧-D-半乳糖盐酸盐通过防止COX-2 N-糖基化并以蛋白酶体依赖的方式增加COX-2蛋白的转换,特异性地抑制COX-2。
应用2-氨基-2-脱氧-D-半乳糖盐酸盐又称为D-半乳糖胺盐酸盐,是肝细胞核苷代谢干扰剂。它能够持续性地损伤肝细胞,主要用于肝脏病理和生化的研究。通过使用该物质复制的肝炎模型,可以接近人体肝炎的病理改变,因此对于筛选和研究抗肝炎药物具有较高的可靠性和实用性。
生物活性半乳糖胺(D-GalN)由半乳糖合成,是一种潜在的肝毒性剂。当与脂多糖结合时,会导致致命的肝损伤和肝功能衰竭,并使细胞对细胞因子,特别是肿瘤坏死因子(TNF) 的毒性作用变得敏感。
制备一种D-半乳糖胺盐酸盐的制备工艺如下:在带有回流冷凝管、搅拌器和温度计的1000ml三口烧瓶中加入500ml无水乙醇,在室温下搅拌并分别加入100g二甲基十二十四烷基胺、500g固载相氧化硅。30分钟内升温至110℃,保持回流反应5小时后降至29℃,经过滤、烘干,得到589g有机胺固载催化剂。
在另一个带有搅拌器和温度计的1000ml三口烧瓶中分别加入190g 5-甲基色胺盐酸盐、600ml二甲基酰胺。然后依次加入59g上述有机胺固载催化剂和150g氯丙烯,在室温条件下反应八小时。将反应液过滤,滤液蒸干后用1150ml异丙醇溶解,并加入89ml 36wt%盐酸进行盐酸化处理,最终得到2-氨基-2-脱氧-D-半乳糖盐酸盐白色晶体130.32g,收率42.05%。
用途用于生化研究。作为测定芳胺N-甲基转移酶和组织均浆中单胺氧化酶活力的底物。
反应信息
-
作为反应物:描述:2-氨基-2-脱氧-D-半乳糖盐酸盐 在 copper(ll) sulfate pentahydrate 、 碳酸氢钠 、 triflic azide 作用下, 以 水 、 甲醇 为溶剂, 生成 2-叠氮基-2-脱氧-D-半乳糖参考文献:名称:豇豆花叶病毒衣壳:用于开发基于碳水化合物的抗肿瘤疫苗的有前途的载体。摘要:针对肿瘤细胞表面碳水化合物的免疫疗法是一种有前途的癌症治疗方法。然而,碳水化合物的低免疫原性提出了巨大的挑战。我们在这里描述了通过豇豆花叶病毒(CPMV)衣壳表面的有序展示来增强碳水化合物的免疫原性。 Tn 聚糖在许多癌细胞表面过度表达,被选为我们研究的模型抗原。此前已有研究表明,针对不同载体上以多种方式呈现的单体形式的 Tn,很难诱导出强烈的 T 细胞依赖性免疫反应。在这项研究中,我们首先合成了由马来酰亚胺或溴乙酰胺部分衍生的 Tn 抗原,该部分选择性地与 CPMV 的半胱氨酸突变体缀合。然后将糖缀合物注射到小鼠体内,并通过酶联免疫吸附测定法测量免疫前和免疫后小鼠血清中的抗体水平。在免疫后第 35 天的血清中获得了高总抗体滴度,更重要的是,高 Tn 特异性 IgG 滴度,表明糖缀合物诱导了 T 细胞依赖性抗体同种型转换。生成的抗体能够识别 MCF-7 乳腺癌细胞和多药耐药乳腺癌细胞系 NCI-ADRDOI:10.1002/chem.200800203
文献信息
-
Low molecular weight polyglucosamines and polygalactosamines申请人:Sabesan Subramaniam公开号:US20060286149A1公开(公告)日:2006-12-21Compositions containing enriched populations of beta linked low molecular weight polymers of galactosamine and glucosamine are provided. The compositions are useful, for example, as antibacterial additives for food and other edible applications, as precursors for synthesizing other biologically active molecules related to chitin/chitosan oligomers, and/or as pharmaceutical compounds.
-
[EN] CARBOHYDRATE CONJUGATED RNA AGENTS AND PROCESS FOR THEIR PREPARATION<br/>[FR] AGENTS D'ARN CONJUGUÉS À DES GLUCIDES ET LEUR PROCÉDÉ DE PRÉPARATION申请人:ALNYLAM PHARMACEUTICALS INC公开号:WO2014025805A1公开(公告)日:2014-02-13This disclosure relates to an improved process for the preparation of carbohydrate conjugates. The disclosure also relates to carbohydrate conjugated iRNA agents comprising these carbohydrate conjugates, which have improved purity and are advantageous for the in vivo delivery of the iRNA agents.
-
Amino Sugar-Bound Anti-Cancerous Noble Metal Complex申请人:NATIONAL UNIVERSITY CORPORATION NARA INSTITUTE OF SCIENCE AND TECHNOLOGY公开号:US20140378402A1公开(公告)日:2014-12-25Disclosed is a pharmaceutical composition containing as an active ingredient a compound represented by formula (I) or a physiologically acceptable salt thereof, and a method for treating cancer, the method including administering the compound represented by formula (I) or the physiologically acceptable salt thereof.公开了一种包含式(I)化合物或其生理可接受盐作为活性成分的药物组合物,以及一种用于治疗癌症的方法,该方法包括施用式(I)化合物或其生理可接受的盐。
-
MEDICINAL PREPARATION申请人:TORAY INDUSTRIES, INC.公开号:EP1787661A1公开(公告)日:2007-05-23A pharmaceutical preparation has a ligand structure specifically recognizing a target site and an amphiphilic compound having a hydrophobic or amphiphilic group. The pharmaceutical preparation employs an amphiphilic compound of specific structure obtained by introducing a chained hydrophilic group with an appropriate flexibility, and thus becomes a fine particle suited for drug targeting. The pharmaceutical preparation is expected to give a prolonged pharmacological effect. A particulate preparation exhibiting a remarkable site targeting property can be formed. Further, according to the selection of matrix forming material, the drug releasing property can be controlled.
-
[EN] TARGETED PLASMA PROTEIN DEGRADATION<br/>[FR] DÉGRADATION CIBLÉE DE PROTÉINES DE PLASMA申请人:NOVARTIS AG公开号:WO2021156792A1公开(公告)日:2021-08-12The present invention is directed to the bifunctional compounds and the use of such bifunctional compounds to lower plasma levels of extracellular target molecules by lysosomal degradation. Such bifunctional compounds have a cell surface receptor ligand covalently linked to a ligand that is capable of binding to an extracellular target molecule (such as a ligand for a growth factor, a cytokine, a chemokine, a hormone, a neurotransmitter, a capsid, a soluble receptor, an extracellular secreted protein, an antibody, a lipoprotein, an exosome, a virus, a cell, or a plasma membrane protein), where the cell surface receptor is associated with receptor mediated endocytosis, including asialoglycoprotein receptor (ASGPR) mediated lysosomal degradation and mannose-6-phosphate (M6PR) mediated lysosomal degradation. Pharmaceutical compositions comprising such bifunctional compounds and methods of treating a disease or disorder mediated by an extracellular molecule using such bifunctional compounds are also provided herein.
表征谱图
-
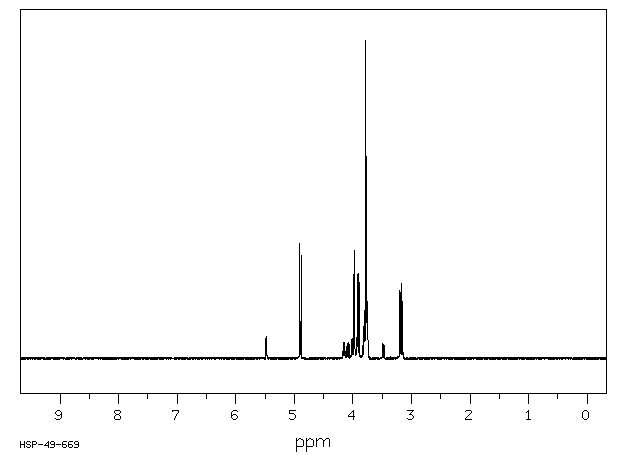
氢谱1HNMR
-
质谱MS
-
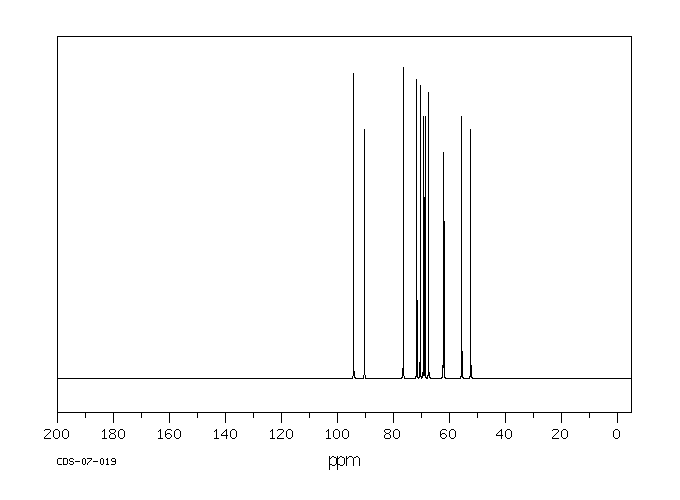
碳谱13CNMR
-
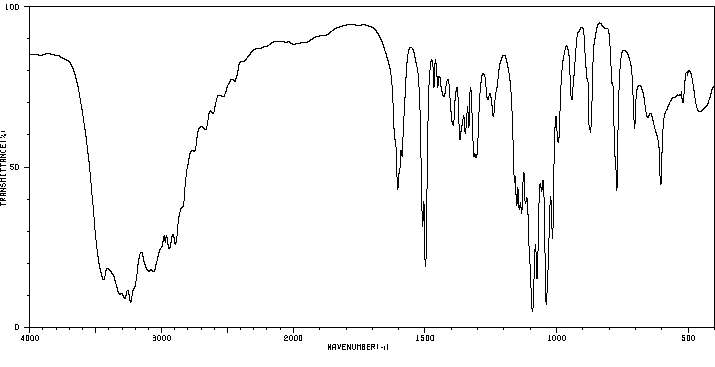
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷