3,4-二羟基苄醇 | 3897-89-0
物质功能分类
中文名称
3,4-二羟基苄醇
中文别名
3,4-二羟基苯甲醇
英文名称
4-(hydroxymethyl)benzene-1,2-diol
英文别名
3,4-dihydroxybenzyl alcohol;4-(hydroxymethyl)catechol;3,4-dihydroxylbenzyl alcohol;3,4-Dihydroxy-benzylalkohol;3,4-Dihydroxy hydroxymethyl benzene
CAS
3897-89-0
化学式
C7H8O3
mdl
——
分子量
140.139
InChiKey
PCYGLFXKCBFGPC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:117°C
-
沸点:362.8±27.0 °C(Predicted)
-
密度:1.399±0.06 g/cm3(Predicted)
-
溶解度:可溶于DMSO(少许)、甲醇(少许)
-
稳定性/保质期:
在常温常压下稳定,应避光存放在通风干燥处,并密封保存。
计算性质
-
辛醇/水分配系数(LogP):0.1
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.14
-
拓扑面积:60.7
-
氢给体数:3
-
氢受体数:3
安全信息
-
危险类别码:R36/37/38
-
海关编码:2907299090
-
安全说明:S26,S36
-
危险性防范说明:P264,P280,P302+P352+P332+P313+P362+P364,P305+P351+P338+P337+P313
-
危险性描述:H315,H319
-
储存条件:常温、避光、通风干燥处,密封保存。
SDS
3,4-二羟基苄醇 修改号码:2
模块 1. 化学品
产品名称: 3,4-Dihydroxybenzyl Alcohol
修改号码: 2
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 3,4-二羟基苄醇
百分比: >96.0%(GC)
CAS编码: 3897-89-0
俗名: 4-Hydroxymethylpyrocatechol , α,3,4-Trihydroxytoluene
分子式: C7H8O3
3,4-二羟基苄醇 修改号码:2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。冷冻储存。
存放于惰性气体环境中。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-浅红黄色
气味: 无资料
3,4-二羟基苄醇 修改号码:2
模块 9. 理化特性
pH: 无数据资料
熔点: 117°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度: 无资料
模块 10. 稳定性和反应性
稳定性: 一般情况下稳定。
反应性: 未报道特殊反应性。
避免接触的条件: 热敏, 气敏
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
3,4-二羟基苄醇 修改号码:2
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布): 针对危险化学品的安全使用、生产、储存、运输、装
卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 3,4-Dihydroxybenzyl Alcohol
修改号码: 2
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 3,4-二羟基苄醇
百分比: >96.0%(GC)
CAS编码: 3897-89-0
俗名: 4-Hydroxymethylpyrocatechol , α,3,4-Trihydroxytoluene
分子式: C7H8O3
3,4-二羟基苄醇 修改号码:2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。冷冻储存。
存放于惰性气体环境中。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-浅红黄色
气味: 无资料
3,4-二羟基苄醇 修改号码:2
模块 9. 理化特性
pH: 无数据资料
熔点: 117°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度: 无资料
模块 10. 稳定性和反应性
稳定性: 一般情况下稳定。
反应性: 未报道特殊反应性。
避免接触的条件: 热敏, 气敏
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
3,4-二羟基苄醇 修改号码:2
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布): 针对危险化学品的安全使用、生产、储存、运输、装
卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-羟基-4-甲氧基苯甲醇 3-hydroxy-4-methoxybenzyl alcohol 4383-06-6 C8H10O3 154.166 4-羟基-3-甲氧基苄醇 4-hydroxymethyl-2-methoxyphenol 498-00-0 C8H10O3 154.166 3,4-二羟基甲苯 4-Methylcatechol 452-86-8 C7H8O2 124.139 原儿茶酸 3,4-Dihydroxybenzoic acid 99-50-3 C7H6O4 154.122 对羟基苯甲醇 (4-hydroxyphenyl)methanol 623-05-2 C7H8O2 124.139 3,4-二羟基苯甲酸甲酯 3,4-dihydroxybenzoic acid methyl ester 2150-43-8 C8H8O4 168.149 3,4-二羟基苯甲醛 3,4-dihydroxybenzaldehyde 139-85-5 C7H6O3 138.123 藜芦酸 Veratric acid 93-07-2 C9H10O4 182.176 3,4-二(苄氧基)苄醇 3,4-bis(benzyloxy)benzyl alcohol 1699-58-7 C21H20O3 320.388 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3-羟基-4-甲氧基苯甲醇 3-hydroxy-4-methoxybenzyl alcohol 4383-06-6 C8H10O3 154.166 4-羟基-3-甲氧基苄醇 4-hydroxymethyl-2-methoxyphenol 498-00-0 C8H10O3 154.166 3,4-二羟基甲苯 4-Methylcatechol 452-86-8 C7H8O2 124.139 原儿茶酸 3,4-Dihydroxybenzoic acid 99-50-3 C7H6O4 154.122 —— 3,4-dihydroxybenzyl acetate (3,4-dihydroxybenzyl acetate) 68292-30-8 C9H10O4 182.176 3,4-二羟基苯甲醛 3,4-dihydroxybenzaldehyde 139-85-5 C7H6O3 138.123 3,4-二羟基苯腈 3,4-dihydroxybenzonitrile 17345-61-8 C7H5NO2 135.122 —— 4-chloromethyl-1,2-dihydroxybenzene —— C7H7ClO2 158.584 3,4-二羟基苯甲酸乙酯 Ethyl protocatechuate 3943-89-3 C9H10O4 182.176
反应信息
-
作为反应物:参考文献:名称:高度可回收的钯离子取代二氧化钛作为多功能无配体催化剂用于醇的选择性氧化和硝基芳烃的还原摘要:采用溶液燃烧法合成了一种贵金属离子催化剂Ti 0.97 Pd 0.03 O 1.97 ,平均晶体尺寸为5 nm。在醇氧化和芳族硝基还原反应中都探索了催化剂的稳定性。该催化剂可有效地将伯醇选择性氧化为醛。在很短的反应时间内实现了各种醇向羰基化合物的有效转化。在 NaBH 4和 H 2存在下进行多种芳香族硝基化合物的还原作为氢源。无溶剂还原也以良好的收率进行。本催化剂在羰基的存在下也更有利地显示出对硝基还原的选择性。通过在氧化和还原反应中轻松使用涂有催化剂的堇青石整料进行回收,最多 10 个循环。 图形概要 简介: Ti 0.97 Pd 0.03 O 1.97催化剂采用溶液燃烧法合成,结晶为锐钛矿相,平均微晶尺寸为5 nm。该催化剂对醇的选择性氧化和硝基芳烃的还原具有高活性。涂覆在堇青石整料上的催化剂甚至可以循环使用多达 10 个循环。DOI:10.1007/s12039-022-02089-3
-
作为产物:描述:参考文献:名称:硫醇在愈创木酚衍生物的生物催化去甲基化中充当甲基陷阱摘要:甲基苯基醚的去甲基化具有挑战性,特别是当产品是容易发生后续反应的儿茶酚衍生物时。对于生物催化去甲基化,先前已经描述过单加氧酶需要分子氧,这可能导致氧化副反应。在这里,我们表明,通过使用钴胺素依赖性甲基转移酶,利用 3-巯基丙酸乙酯等硫醇作为甲基捕获物,可以对此类化合物进行厌氧去甲基化。仅使用两当量的该试剂,即可对多种取代愈创木酚衍生物进行去甲基化,转化率大多高于 90%。该策略用于以 97% 的分离收率制备一克规模的高价值抗氧化剂羟基酪醇。DOI:10.1002/anie.202104278
文献信息
-
Reduction of Carbonyl Compounds with NaBH<sub>4</sub> under Ultrasound Irradiation and Aprotic Condition作者:Behzad Zeynizadeh、Saiedeh YahyaeiDOI:10.1515/znb-2004-0612日期:2004.6.1
A variety of carbonyl compounds are reduced to their corresponding alcohols with sodium borohydride under ultrasound irradiation and aprotic condition. Reduction reactions are performed in THF at room temperature or under reflux condition. The product alcohols were obtained in good to excellent yields. The chemoselective reduction of aldehydes over ketones was achieved successfully with this system.
-
Modified Hydroborate Agent: (2,2′-Bipyridyl)(tetrahydroborato)zinc Complex, [Zn(BH<sub>4</sub>)<sub>2</sub>(bpy)], as a New, Stable, Efficient Ligand-Metal Hydroborate and Chemoselective Reducing Agent作者:Behzad ZeynizadehDOI:10.1246/bcsj.76.317日期:2003.2rato)zinc complex, [Zn(BH4)2(bpy)], is a new white stable compound which has been used for efficient reduction of variety of carbonyl compounds such as aldehydes, ketones, acyloins, α-diketones and α,β-unsaturated carbonyl compounds (1,2-reduction) to their corresponding alcohols in acetonitrile at room temperature. Excellent chemoselectivity was also observed for the reduction of aldehydes over ketones
-
Oxidative esterification via photocatalytic C–H activation作者:Sanny Verma、R. B. Nasir Baig、Changseok Han、Mallikarjuna N. Nadagouda、Rajender S. VarmaDOI:10.1039/c5gc02025e日期:——
Direct oxidative esterification of alcohol
via photocatalytic C–H activation has been developed using VO@g-C3N4 catalyst; expeditious esterification of alcohol occurs under neutral conditions using visible light.直接氧化酯化醇通过光催化C-H活化已经开发出来,使用VO@g-C3N4催化剂;在中性条件下利用可见光迅速进行醇的酯化。 -
Temperature-Directed Biocatalysis for the Sustainable Production of Aromatic Aldehydes or Alcohols作者:Jun Ni、Yan-Yan Gao、Fei Tao、Hong-Yu Liu、Ping XuDOI:10.1002/anie.201710793日期:2018.1.26The biosynthesis of aromatic aldehydes and alcohols from renewable resources is currently receiving considerable attention because of an increase in demand, finite fossil resources, and growing environmental concerns. Here, a temperature‐directed whole‐cell catalyst was developed by using two novel enzymes from a thermophilic actinomycete. Ferulic acid, a model lignin derivative, was efficiently converted
-
Lanthanoids in Organic Synthesis. I. The Novel Reduction of Carboxylic Acids with Samarium Diiodide-Base System作者:Yasuko Kamochi、Tadahiro KudoDOI:10.1246/bcsj.65.3049日期:1992.11were rapidly reduced with samarium diiodide by the addition of base in the presence of protic solvent at room temperature to the corresponding alcohols. Sodium benzoate was similarly reduced with samarium diiodide in the presence of H2O in a good yield. In the similar reactions of benzoic acid derivatives bearing carboxyl, formyl, carbamoyl, methoxyl, and chloro groups, these functional groups were
表征谱图
-
氢谱1HNMR
-
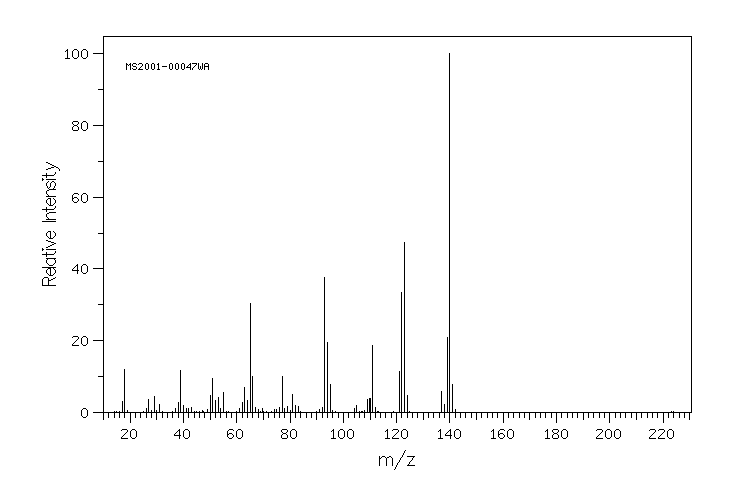
质谱MS
-
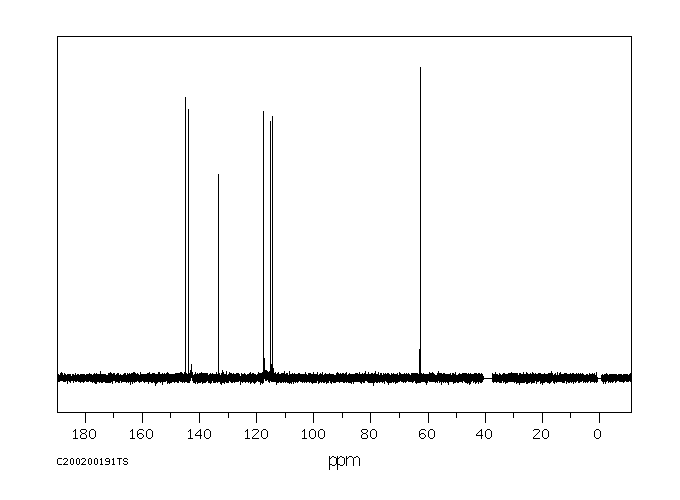
碳谱13CNMR
-
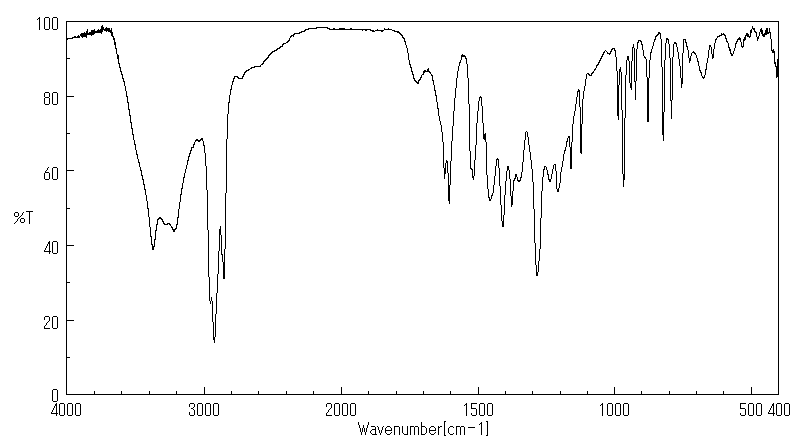
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-氯-6-羟基苯基)硼酸
黄柄曲菌素
高香草酸-d3
高香草酸-13C6
高香草酸
高香兰酸乙酯
高辣椒素II
高二氢辣椒素I
香草醛醛肟
香草醛苯腙
香草醛-甲氧基-13C
香草醛-(N-对甲苯基肟)
香草醛
香草酸肼
香草壬酰胺
香草基扁桃酸乙酯
香草吗啉
香草二乙胺
香兰素胺硬脂酸盐
香兰素胺硬脂酸盐
香兰素胺盐酸盐
香兰素丙二醇缩醛
香兰素13C6
香兰素-D3
香兰基乙基醚
香兰基丁醚
顺式-5-正十五碳-8'-烯基间苯二酚
顺式-1-(2-羟基-5-甲基苯基)-2-丁烯-1-酮
顺式-1-(2-羟基-4-甲氧基苯基)-2-丁烯-1-酮
顺-3-氯二氢-5-苯基呋喃-2(3H)-酮
雌二醇杂质1
降二氢辣椒碱
阿诺洛尔
阿瓦醇
阿普斯特杂质
间苯二酚双(二苯基磷酸酯)
间苯二酚-烯丙醇聚合物
间苯二酚-D6
间苯二酚
间苯三酚甲醛
间苯三酚二水合物
间苯三酚
间羟基苯乙基溴
间硝基苯酚
间甲酚紫钠盐
间甲酚与对甲酚和苯酚甲醛树脂的聚合物
间甲酚-D7
间甲酚-D3
间甲酚
间溴苯酚