3-羟基-4-甲氧基苯甲醇 | 4383-06-6
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:135-137 °C (lit.)
-
沸点:315.8±27.0 °C(Predicted)
-
密度:1.226±0.06 g/cm3(Predicted)
-
溶解度:溶于甲醇
-
保留指数:1477.9
-
稳定性/保质期:
在常温常压下,它是一种稳定的类白色结晶粉末。
计算性质
-
辛醇/水分配系数(LogP):0.7
-
重原子数:11
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:49.7
-
氢给体数:2
-
氢受体数:3
安全信息
-
危险品标志:Xi
-
安全说明:S22,S24/25
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2909500000
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:请将药品存放在避光、通风干燥的地方,并密封保存。
SDS
模块 1. 化学品
1.1 产品标识符
: 3-羟基-4-甲氧基苯甲醇
产品名称
1.2 鉴别的其他方法
Isovanillyl alcohol
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
根据全球协调系统(GHS)的规定,不是危险物质或混合物。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: Isovanillyl alcohol
别名
: C8H10O3
分子式
: 154.16 g/mol
分子量
无
模块 4. 急救措施
4.1 必要的急救措施描述
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。
皮肤接触
用肥皂和大量的水冲洗。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。
4.2 主要症状和影响,急性和迟发效应
取决于接触的时间和强度。程度从轻度刺激到严重组织损伤不等。, 长期或频繁接触会导致:, 损害眼睛,
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
避免粉尘生成。 避免吸入蒸气、烟雾或气体。
6.2 环境保护措施
不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
扫掉和铲掉。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
个体防护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所选择身体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 结晶
颜色: 白色
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 135 - 137 °C - lit.
f) 沸点、初沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
酸, 酰基氯, 酸酐, 氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞致突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 通过皮肤吸收可能有害。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
取决于接触的时间和强度。程度从轻度刺激到严重组织损伤不等。, 长期或频繁接触会导致:, 损害眼睛,
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国运输名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3,4-二甲氧基苄醇 (3,4-dimethoxyphenyl)methanol 93-03-8 C9H12O3 168.192 2-甲氧基-5-甲基苯酚 5-methyl-2-methoxyphenol 1195-09-1 C8H10O2 138.166 3,4-二羟基苄醇 4-(hydroxymethyl)benzene-1,2-diol 3897-89-0 C7H8O3 140.139 胡椒醇 piperonol 495-76-1 C8H8O3 152.15 异香兰素 isovanillin 621-59-0 C8H8O3 152.15 3-苄氧基-4-甲氧苄醇 3-benzyloxy-4-methoxy-benzyl alcohol 1860-60-2 C15H16O3 244.29 3-苄氧基-4-甲氧基苯甲醛 3-benzyloxy-4-methoxybenzaldehyde 6346-05-0 C15H14O3 242.274 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-羟基-3-甲氧基苄醇 4-hydroxymethyl-2-methoxyphenol 498-00-0 C8H10O3 154.166 —— 3-Hydroxy-4-methoxybenzylphenylether 58451-96-0 C14H14O3 230.263 3,4-二羟基苄醇 4-(hydroxymethyl)benzene-1,2-diol 3897-89-0 C7H8O3 140.139 3-羟基-4-甲氧基苯甲酸 Isovanillic acid 645-08-9 C8H8O4 168.149 —— 3-hydroxy-4-methoxy-benzyl acetate 63867-04-9 C10H12O4 196.203 —— 2-methoxy-4-[(methoxymethoxy)methyl]phenol 1058649-06-1 C10H14O4 198.219 —— (3-isopropoxy-4-methoxyphenyl)methanol 518034-14-5 C11H16O3 196.246 —— O-allylisovanillyl alcohol 854954-43-1 C11H14O3 194.23 3-羟基-4-甲氧基苯甲酸甲酯 3-hydroxy-4-methoxybenzoate 6702-50-7 C9H10O4 182.176 异香兰素 isovanillin 621-59-0 C8H8O3 152.15 3-(3-甲氧基丙氧基)-4-甲氧基苄醇 (4-methoxy-3-(3-methoxypropoxy)phenyl)methanol 172900-74-2 C12H18O4 226.273 3-苄氧基-4-甲氧苄醇 3-benzyloxy-4-methoxy-benzyl alcohol 1860-60-2 C15H16O3 244.29 —— 3-hydroxy-4-methoxybenzyl bromide 111394-51-5 C8H9BrO2 217.062 —— 3-hydroxy-4-methoxybenzyl benzoate —— C15H14O4 258.274 3-乙酰氧基-4-甲氧基苄醇 3-acetoxy-4-methoxybenzyl alcohol 63867-05-0 C10H12O4 196.203 —— bis(3-hydroxy-4-methoxyphenyl)methane 344762-21-6 C15H16O4 260.29 —— 8-methylnonanoic acid (3-hydroxy-4-methoxyphenyl)methyl ester 1000377-97-8 C18H28O4 308.418 3-环戊氧基-4-甲氧苄醇 (3-cyclopentyloxy-4-methoxyphenyl)methanol 133332-49-7 C13H18O3 222.284 —— 2-methoxy-5-phenethylphenol 36868-37-8 C15H16O2 228.291 —— m,p'-bisguaiacol F —— C15H16O4 260.29 —— 2-methoxy-5-((tetrahydro-2H-pyran-2-yloxy)methyl)phenol —— C13H18O4 238.284 —— 3-hydroxy-4-methoxybenzamide —— C8H9NO3 167.164 —— [4-Methoxy-3-(4-trifluoromethyl-benzyloxy)-phenyl]-methanol 613240-66-7 C16H15F3O3 312.289 —— 5-acetoxymethyl-2-methoxyphenyl acetate 63866-99-9 C12H14O5 238.24 2-苄氧基-4-溴甲基-1-甲氧基苯 4-methoxy-3-(benzyloxy)benzyl bromide 55667-12-4 C15H15BrO2 307.187 4-(氯甲基)-1-甲氧基-2-苯基甲氧基苯 3-benzyloxy-4-methoxy-benzyl chloride 1699-38-3 C15H15ClO2 262.736 石斛酚 gigantol 67884-30-4 C16H18O4 274.317 5-[2-(3,5-二甲氧基苯基)乙基]-2-甲氧基苯酚 3'-hydroxy-3,4',5-trimethoxydihydrostilbene 71135-71-2 C17H20O4 288.343 - 1
- 2
- 3
反应信息
-
作为反应物:描述:3-羟基-4-甲氧基苯甲醇 在 2,2,6,6-四甲基哌啶氧化物 、 potassium carbonate 、 C21H22Br2CuN2S 作用下, 以 水 为溶剂, 以99 %的产率得到异香兰素参考文献:名称:空气稳定性铜(II)配合物催化生物质模型化合物藜芦醇在水中的选择性好氧氧化摘要:合成了分别具有NNS、N(NH)S和NNO配体骨架的空气稳定的四角锥形溴化铜(II)配合物1、2和3,并成功用作生物质模型化合物藜芦醇有氧氧化的有效催化剂。水作为一种绿色、可持续的反应介质。综合体2表现出了最好的催化活性。氧化反应在催化量的铜催化剂和 TEMPO ((2,2,6,6-四甲基哌啶-1-基)氧基) 存在下,利用空气作为可持续氧化剂,在环境条件 (40 °C) 下成功进行。需要添加外部碱以获得定量产率和对所需醛产物的完全选择性。氧化反应也可以在没有碱存在下进行;然而,随着过度氧化产物藜芦酸的形成,选择性下降。乙腈是氧化反应中最常用的溶剂,甲醇、乙醇、丙酮和乙酸乙酯等各种绿色溶剂也用于同样的目的。尽管乙腈和水具有相似的催化效率,我们在考虑绿色和可持续方面后选择了水。利用优化的水中反应条件将其他木质素生物质衍生的醇以及各种其他取代的苄醇氧化成相应的醛。通过简单的后处理过程(过滤和蒸发DOI:10.1039/d3cy00671a
-
作为产物:参考文献:名称:高效的Meerwein-Ponndorf-Verley降低了强大的锆-有机硼酸混合溶液摘要:Meerwein-Ponndorf-Verley(MPV)反应是一种选择性还原羰基的有吸引力的方法,而先进催化剂的设计是这类有趣反应的关键。本文中,我们使用1,4-苯二硼酸(BDB)作为MPV还原的前体,制备了新型有机硼酸锆。制备的Zr-BDB对MPV还原各种生物质衍生的羰基化合物具有优异的催化性能(即,乙酰丙酸酯,醛和酮)。更重要的是,由于硼酸根在氢源中对羟基的活化作用,配体上硼酸根的数目显着影响Zr-有机配体杂化物的催化活性。详细的研究表明,Zr-BDB的优异性能归功于Zr 4+和硼酸盐的协同作用。值得注意的是,这是提高硼酸根基MPV反应中Zr基催化剂活性的第一项工作。DOI:10.1039/d0gc04179c
文献信息
-
[EN] A PROCESS FOR THE PREPARATION OF INTERMEDIATES USEFUL IN THE PRODUCTION OF ALISKIREN<br/>[FR] PROCÉDÉ DE PRÉPARATION D'INTERMÉDIAIRES UTILES DANS LA PRODUCTION DE L'ALISKIREN申请人:WATSON LAB INC公开号:WO2012078147A1公开(公告)日:2012-06-14A process for preparing 4-[(2R)-2-X-methyl)-3-methylbutyl]-1 -methoxy-2-3(3- methoxypropoxy)-benzene wherein X is a leaving group and use of this compound in the synthesis of aliskiren.一种制备4-[(2R)-2-X-甲基)-3-甲基丁基]-1-甲氧基-2-3(3-甲氧基丙氧基)-苯的方法,其中X是一个离去基团,并且利用该化合物合成阿利司琴。
-
A two-phase system for the clean and high yield synthesis of furylmethane derivatives over –SO<sub>3</sub>H functionalized ionic liquids作者:S. H. Shinde、C. V. RodeDOI:10.1039/c7gc01654a日期:——respective ionic liquids. Among the several preapered ionic liquids, strong acidic imidazolium based butylsulfonic acid (6) showed the best activity with a maximum of 84% yield of condensation product. This strategy offers significantly high yield production of condensation products of furan and furfural as compared to the traditional mineral acid route. The activity and stability of the -SO3H functionalized我们在这里报告了一种新的有效的独特的两相反应系统,用于从糠醛和呋喃中高产率生产三(糠基)甲烷。该策略包括酸性水相(水+ -SO3H官能化的IL)和呋喃相,它们显着抑制了聚合物的形成,从而提高了三(呋喃基)甲烷的收率。呋喃既用作反应物又用作萃取溶剂,以回收缩合产物。为了进行比较,制备了不同的-SO3H官能化离子液体,并评估了它们在呋喃和糠醛缩合中的性能。发现具有烷基链接头的离子液体比咪唑鎓连接的N-磺酸更有效和酸性。除此之外,咪唑/三乙胺/吡啶和-SO3H之间的碳链长度增加,增加了相应离子液体的催化活性。在几种预离子化的液体中,强酸性咪唑基丁基磺酸(6)表现出最佳的活性,缩合产物的收率最高为84%。与传统的无机酸路线相比,该策略可显着提高呋喃和糠醛缩合产物的收率。-SO3H功能化的IL的活性和稳定性通过其成功的七个循环而得到证实,而没有丧失活性。然后,成功地扩展了这一新策略,使呋喃衍生物(例
-
Macrocyclic Compounds Useful as Bace Inhibitors申请人:Betschart Claudia公开号:US20080132477A1公开(公告)日:2008-06-05The invention relates to novel macrocyclic compounds of the formula (I), in which all of the variables are as defined in the specification, the number of ring atoms included in the macrocyclic ring being 14, 15, 16 or 17, in free base form or in acid addition salt form, to their preparation, to their use as medicaments and to medicaments comprising them.本发明涉及具有公式(I)的新型大环化合物,其中所有变量如说明书中所定义,大环环中包含的环原子数为14、15、16或17,以自由碱形式或酸加成盐形式存在,以及它们的制备方法、作为药物的使用以及包含它们的药物。
-
Reusable Co-nanoparticles for general and selective <i>N</i>-alkylation of amines and ammonia with alcohols作者:Zhuang Ma、Bei Zhou、Xinmin Li、Ravishankar G. Kadam、Manoj B. Gawande、Martin Petr、Radek Zbořil、Matthias Beller、Rajenahally V. JagadeeshDOI:10.1039/d1sc05913k日期:——A general cobalt-catalyzed N-alkylation of amines with alcohols by borrowing hydrogen methodology to prepare different kinds of amines is reported. The optimal catalyst for this transformation is prepared by pyrolysis of a specific templated material, which is generated in situ by mixing cobalt salts, nitrogen ligands and colloidal silica, and subsequent removal of silica. Applying this novel Co-nanoparticle-based
-
Isolation, structure, and synthesis of combretastatin A-2, A-3, and B-2作者:George R. Pettit、Sheo Bux SinghDOI:10.1139/v87-399日期:1987.10.1
Further investigation of the South African tree Combretumcaffrum (Combretaceae) for murine P388 lymphocytic leukemia (PS) cell-growth inhibitory substances has led to discovery of three new active constituents designated combretastatins A-2 (5a, PS ED50 0.027 μg/mL), A-3 (5b, PS ED50 0.026 μg/mL), and B-2 (3b, PS ED50 0.32 μg/mL). Both combretastatins A-2 and A-3 were found to markedly inhibit tubulin polymerization. The structure of each combretastatin was firmly established by a combination of high resolution (400 MHz) 1H and 13C nuclear magnetic resonance and mass spectral analyses followed by total syntheses. The conversion of methyl gallate (7b) to combretastatin A-2 via intermediates 7c → 7d → 7e → 7a and 6a → 5a illustrates the practical synthetic route utilized for obtaining these substances. The Wittig reaction employed as the penultimate step in obtaining combretastatins A-3, afforded predominantly the natural Z isomer.
对南非树木Combretum caffrum(锥花科)进行进一步研究,以寻找对小鼠P388淋巴细胞白血病(PS)细胞生长具有抑制作用的物质,发现了三种新的活性成分,分别命名为combretastatin A-2(5a,PS ED50 0.027 μg/mL)、A-3(5b,PS ED50 0.026 μg/mL)和B-2(3b,PS ED50 0.32 μg/mL)。发现combretastatin A-2和A-3均明显抑制微管聚合。通过高分辨率(400 MHz)的1H和13C核磁共振和质谱分析以及总合成,确定了每种combretastatin的结构。通过从甲基没食子酸酯(7b)经过中间体7c→7d→7e→7a和6a→5a的转化,说明了用于获得这些物质的实用合成路线。在获得combretastatin A-3的倒数第二步中采用的Wittig反应主要产生了天然的Z异构体。
表征谱图
-
氢谱1HNMR
-
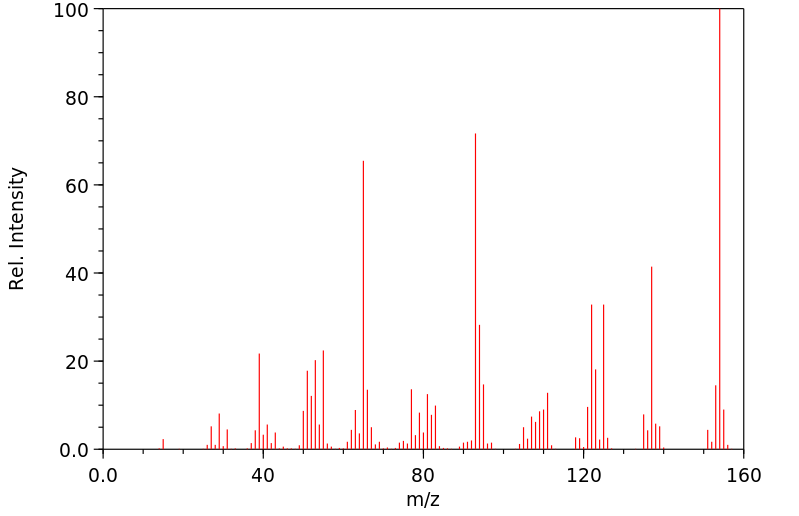
质谱MS
-
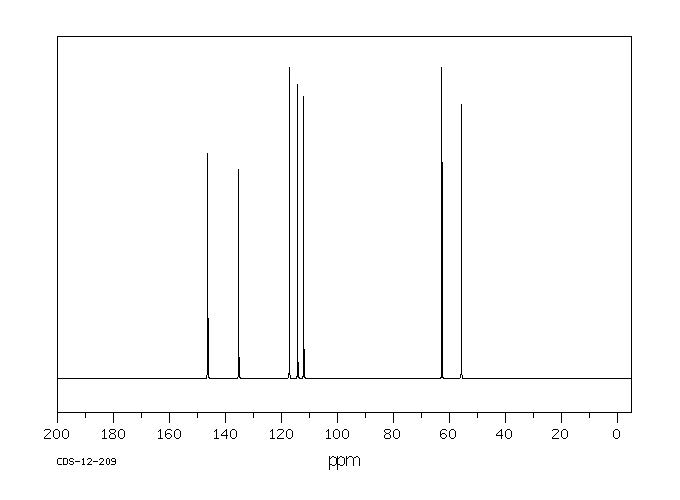
碳谱13CNMR
-
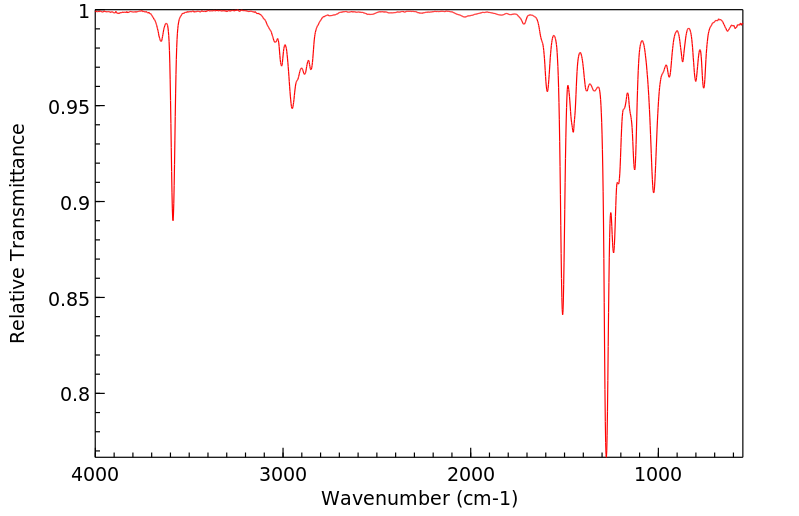
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息