甲基丁二酸二甲酯 | 1604-11-1
中文名称
甲基丁二酸二甲酯
中文别名
2-甲基琥珀酸二甲酯
英文名称
dimethyl methylsuccinate
英文别名
methyl 2-methylsuccinate;dimethyl 2-methylbutanedioate
CAS
1604-11-1
化学式
C7H12O4
mdl
MFCD00008449
分子量
160.17
InChiKey
NFOQJNGQQXICBY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:196 °C (lit.)
-
密度:1.076 g/mL at 25 °C (lit.)
-
闪点:182 °F
-
保留指数:1043.7;1035;1035
-
稳定性/保质期:
在常温常压下保持稳定
计算性质
-
辛醇/水分配系数(LogP):0.4
-
重原子数:11
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:0.714
-
拓扑面积:52.6
-
氢给体数:0
-
氢受体数:4
安全信息
-
WGK Germany:2
-
海关编码:2917190090
-
储存条件:常温、避光、存于通风干燥处。
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product identifiers
Product name : Dimethyl methylsuccinate
CAS-No. : 1604-11-1
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008.
Not a hazardous substance or mixture according to EC-directives 67/548/EEC or 1999/45/EC.
Label elements
The product does not need to be labelled in accordance with EC directives or respective national laws.
Other hazards - none
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Formula : C7H12O4
Molecular Weight : 160,17 g/mol
Section 4. FIRST AID MEASURES
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Consult a physician.
In case of eye contact
Flush eyes with water as a precaution.
If swallowed
Do NOT induce vomiting. Never give anything by mouth to an unconscious person. Rinse mouth with
water. Consult a physician.
Most important symptoms and effects, both acute and delayed
Indication of any immediate medical attention and special treatment needed
no data available
Section 5. FIREFIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
For small (incipient) fires, use media such as "alcohol" foam, dry chemical, or carbon dioxide. For large
fires, apply water from as far as possible. Use very large quantities (flooding) of water applied as a mist or
spray; solid streams of water may be ineffective. Cool all affected containers with flooding quantities of
water.
Special hazards arising from the substance or mixture
Carbon oxides
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
Use water spray to cool unopened containers.
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Avoid breathing vapors, mist or gas. Remove all sources of ignition. Beware of vapours accumulating to
form explosive concentrations. Vapours can accumulate in low areas.
Environmental precautions
Prevent further leakage or spillage if safe to do so. Do not let product enter drains.
Methods and materials for containment and cleaning up
Contain spillage, and then collect with an electrically protected vacuum cleaner or by wet-brushing and
place in container for disposal according to local regulations (see section 13). Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Avoid inhalation of vapour or mist.
Keep away from sources of ignition - No smoking.Take measures to prevent the build up of electrostatic
charge.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Containers which are
opened must be carefully resealed and kept upright to prevent leakage.
Specific end uses
no data available
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and
at the end of workday.
Personal protective equipment
Eye/face protection
Safety glasses with side-shields conforming to EN166 Use equipment for eye protection tested
and approved under appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
impervious clothing, The type of protective equipment must be selected according to the
concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Where risk assessment shows air-purifying respirators are appropriate use a full-face respirator
with multi-purpose combination (US) or type ABEK (EN 14387) respirator cartridges as a backup
to engineering controls. If the respirator is the sole means of protection, use a full-face supplied air
respirator. Use respirators and components tested and approved under appropriate government
standards such as NIOSH (US) or CEN (EU).
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
a) Appearance Form: liquid
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing no data available
point
f) Initial boiling point and 196 °C - lit.
boiling range
g) Flash point 83 °C - closed cup
h) Evaporation rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density 1,076 g/mL at 25 °C
n) Water solubility no data available
o) Partition coefficient: n- log Pow: 0,755
octanol/water
p) Autoignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
Section 10. STABILITY AND REACTIVITY
Reactivity
no data available
Chemical stability
no data available
Possibility of hazardous reactions
no data available
Conditions to avoid
Heat, flames and sparks.
Incompatible materials
no data available
Hazardous decomposition products
Other decomposition products - no data available
Section 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Potential health effects
Inhalation May be harmful if inhaled. May cause respiratory tract irritation.
Ingestion May be harmful if swallowed.
Skin May be harmful if absorbed through skin. May cause skin irritation.
Eyes May cause eye irritation.
Additional Information
RTECS: Not available
Section 12. ECOLOGICAL INFORMATION
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
no data available
Other adverse effects
no data available
Section 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
This combustible material may be burned in a chemical incinerator equipped with an afterburner and
scrubber. Offer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed
professional waste disposal service to dispose of this material.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
SECTION 15 - REGULATORY INFORMATION
N/A
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 甲基丁二酸 2-methylbutanedioic acid 498-21-5 C5H8O4 132.116 甲基琥珀酸酐 2-methylsuccinic anhydride 4100-80-5 C5H6O3 114.101 2-氰基-3-甲基-丁二酸二乙酯 2-Cyano-3-methylbernsteinsaeure-diethylester 60298-17-1 C10H15NO4 213.233 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 R-2-甲基琥珀酸甲酯 (R)-methylsuccinic acid dimethyl ester 22644-27-5 C7H12O4 160.17 —— (S)-dimethyl 2-methylsuccinate —— C7H12O4 160.17 —— (R)-2-methylsuccinate-1-monomethyl ester 83509-04-0 C6H10O4 146.143 (S)-2-甲基琥珀酸-1-甲酯 (S)-4-methoxy-3-methyl-4-oxobutanoic acid 111266-16-1 C6H10O4 146.143 2-甲基琥珀酸氢甲酯 1-methyl (RS)-methylsuccinate 32980-25-9 C6H10O4 146.143 —— 4-methoxy-2-methyl-4-oxobutanoic acid 23268-03-3 C6H10O4 146.143 (2S)-甲基丁二酸 4-甲酯 (S)-2-Methyl-succinic acid 4-methyl ester 111266-27-4 C6H10O4 146.143 (R)-(+)-3-甲基琥珀酸单甲酯 (R)-2-methyl-succinic acid 4-methyl ester 81025-83-4 C6H10O4 146.143 —— 1-O-methyl 4-O-propyl 2-methylbutanedioate 111195-87-0 C9H16O4 188.224 —— methyl-succinic acid dipropyl ester 56108-32-8 C11H20O4 216.277 甲基丁二酸 2-methylbutanedioic acid 498-21-5 C5H8O4 132.116 (S)-(-)-甲基丁二酸 L-malic acid 2174-58-5 C5H8O4 132.116 4-氯-2-甲基-4-氧代丁酸甲酯 3-Carbomethoxybutyryl chloride 59700-83-3 C6H9ClO3 164.589 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:用于硫醇介导摄取的环状硫代磺酸盐:级联交换剂、转运蛋白、抑制剂摘要:硫醇介导的摄取正在成为一种强大的穿透细胞的方法。环状低硫属元素化物 (COC) 已被确定为能够实现和抑制硫醇介导的摄取的特殊支架,因为它们可以充当动态共价级联交换器,即每次交换都会产生一个新的共价连接交换器。在这项研究中,我们的重点是基本上未开发的具有较高氧化水平的 COC。潜在动态共价交换级联的定量表征表明,即使在低 pH 值下,环状硫代磺酸盐 (CTO) 的初始开环也会高速进行。释放的亚磺酸盐在非质子环境中与二硫化物交换,但在质子环境中则更少。因此引入疏水域以将 CTO 引导到疏水口袋中以增强其反应性。配备了这样的导演组,荧光标记的 CTO 比流行的芦笋酸更有效地进入活细胞的胞质溶胶。作为竞争性药物,CTO 可抑制各种 COC 转运蛋白和 SARS-CoV-2 慢病毒载体的摄取。使用不同转运蛋白发现的正交趋势支持多个细胞伙伴的存在,以解释硫醇介导的摄取的不同表达。二聚体的主要自我抑制和DOI:10.1021/jacsau.1c00573
-
作为产物:参考文献:名称:具有手性二硫醚配体的阳离子铱配合物:氢化条件下的合成、表征和反应性摘要:为了研究硫取代基和金属环尺寸对乙酰氨基丙烯酸酯加氢反应的影响,制备了一系列含有手性二硫醚配体的阳离子 IrI 配合物。在配合物 6、7 和 10 的情况下,由于硫转化过程,在溶液中观察到非对映异构体的混合物。相比之下,通过使用抑制配合物 8 和 9 中 S 反转的双环配体可以有效地控制这种流动行为。 配合物 10b 的固态结构仅显示一种非对映异构体,其中硫取代基处于相对反位和整体SCSSCSSSS 在配位的二硫醚配体上的构型。含有七元和六元金属环 (6b–d, 7b,c, 10a,b) 的铱配合物通过 S-配体取代与底物反应,而这种取代的速率与氟原子在芳环上的位置有关。相反,含有双金属环(8 和 9)的配合物不会被底物置换。根据相应二氢化物配合物(13和14)的高稳定性分析配合物8和9的催化氢化活性。在这两种情况下,四种可能的非对映二氢化物物质中只有两种在溶液中形成。(© Wiley-VCHDOI:10.1002/ejic.200400828
文献信息
-
Asymmetric catalysis based on chiral phospholanes and hydroxyl phospholanes申请人:The Penn State Research Foundation公开号:US06727377B2公开(公告)日:2004-04-27Chiral phosphine ligands derived from chiral natural products including D-mannitol and tartaric acid. The ligands contain one or more 5-membered phospholane rings with multiple chiral centers, and provide high stereoselectivity in asymmetric reactions.
-
CYCLIC HYDROFLUOROETHER COMPOUNDS AND PROCESSES FOR THEIR PREPARATION AND USE申请人:Vitcak Daniel R.公开号:US20070267464A1公开(公告)日:2007-11-22A hydrofluoroether compound comprises at least one five- or six-membered, perfluorinated heterocyclic ring, each ring comprising four or five ring carbon atoms and one or two catenated heteroatoms selected from divalent ether oxygen atoms and trivalent nitrogen atoms, at least one of the catenated heteroatoms being a divalent ether oxygen atom, and each of the ring carbon atoms adjacent to the divalent ether oxygen atom bearing a fluorochemical group that comprises a tetrafluoroethylidene moiety (—(CF 3 )CF—) that is directly bonded to the ring carbon atom, the fluorochemical group optionally comprising at least one catenated heteroatom selected from divalent ether oxygen atoms and trivalent nitrogen atoms.
-
Highly Regioselective Rhodium-Catalysed Hydroformylation of Unsaturated Esters: The First Practical Method for Quaternary Selective Carbonylation作者:Matthew L. Clarke、Geoffrey J. RoffDOI:10.1002/chem.200600914日期:2006.10.25chemoselectivity. Hydroformylation of a range 1,1-di- and 1,1,2-trisubstituted unsaturated esters yields quaternary aldehydes that are forbidden products according to Keulemans Rule. The aldehydes can be reductively aminated with molecular hydrogen to give beta-amino acid esters in high yield. The overall green chemical process involves converting terminal alkynes into unusual beta-amino acid esters with only
-
Enantiomerically Pure Bis(phosphanyl)carbaborane(12) Compounds作者:Sebastian Bauer、Steffen Tschirschwitz、Peter Lönnecke、René Frank、Barbara Kirchner、Matthew L. Clarke、Evamarie Hey‐HawkinsDOI:10.1002/ejic.200900304日期:2009.7Enantiomerically pure (RP,RP)- and (RP,SP)-1,2-bis[1-adamantyloxy-(–)-menthyloxyphosphanyl]-closo-dicarbaborane(12), 1,2-bis[bis(–)-menthyloxyphosphanyl]-closo-dicarbaborane(12)and 1,2-bis[bis(4-tert-butylphenyloxy)phosphanyl]-closo-dicarbaborane(12) were synthesised by the reaction of dilithiated 1,2-dicarba-closo-dodecaborane(12) with two equivalents of the corresponding chlorophosphite. The phosphonites对映体纯 (RP,RP)- 和 (RP,SP)-1,2-双[1-金刚烷氧基-(-)-薄荷氧基膦酰基]-closo-dicarbaborane(12), 1,2-bis[bis(-)-由二锂化的 1,2-二碳-二碳硼烷 (12) 和 1,2-双[双 (4-叔丁基苯氧基) 膦酰基]-邻-二碳硼烷 (12) 反应合成12) 与两当量的相应氯亚磷酸酯。亚膦酸酯对差向异构化、氧气和水稳定。观察到 P…P 空间耦合,3JPP 耦合常数由光谱模拟和 DFT 计算确定。制备了钼和铑的晚期过渡金属配合物来研究双(膦酰基)碳硼烷 (12) 化合物的配位特性。在与各种烯烃的均相催化加氢甲酰化反应中研究了各种铑配合物的催化性能。(© Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, Germany, 2009)
-
[EN] IONIC LIQUID SOLVENTS<br/>[FR] SOLVANTS LIQUIDES IONIQUES申请人:UNIV DUBLIN CITY公开号:WO2010097412A1公开(公告)日:2010-09-02A chiral ionic compound comprising an alkyl substituted imidazolium or pyridinium cationic core having an alkyl ester side chain (-alkyl-C(O)O-) directly linked to the core and an associated counter anion, characterized in that the -O- atom of the ester side chain is linked to an alpha, a beta or a gamma hydroxycarboxylic acid functionality via the alpha, beta or gamma hydroxy of the acid functionality and the hydroxycarboxylic acid functionality has at least one asymmetric carbon, or characterized in that an -N= atom of the alkyl substituted imidazolium or pyridinium cationic core is substituted with an alpha, a beta or a gamma hydroxy group of a alpha, a beta or a gamma hydroxycarboxylic acid functionality and the hydroxycarboxylic acid functionality has at least one asymmetric carbon. The chiral ionic liquids (CILs) may be used as novel solvents, in particular for organic synthesis. The CILs have the potential to induce asymmetry into substrates or catalysts in a variety of organic transformations. A number of the compounds have low antimicrobial and low antifungal toxicities and are also biodegradable CILs.
表征谱图
-
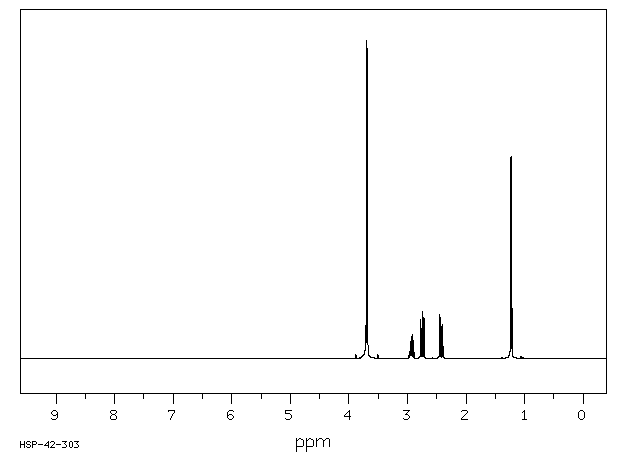
氢谱1HNMR
-
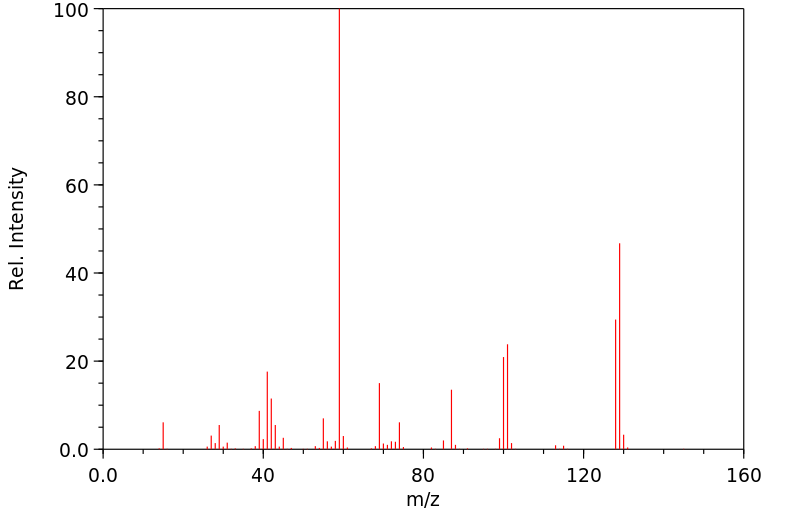
质谱MS
-
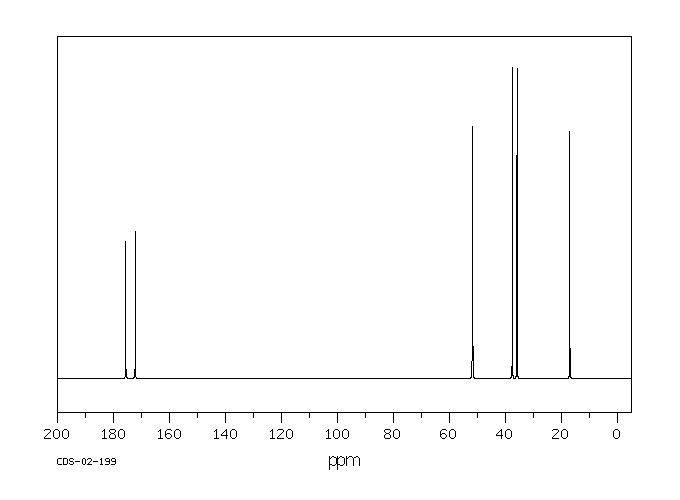
碳谱13CNMR
-
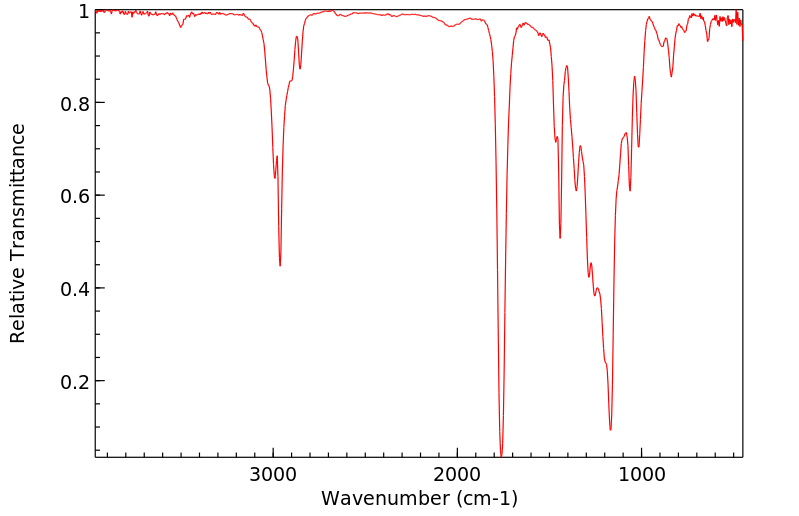
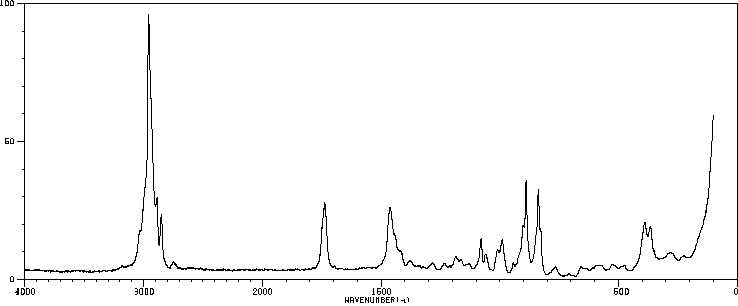
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯