代谢
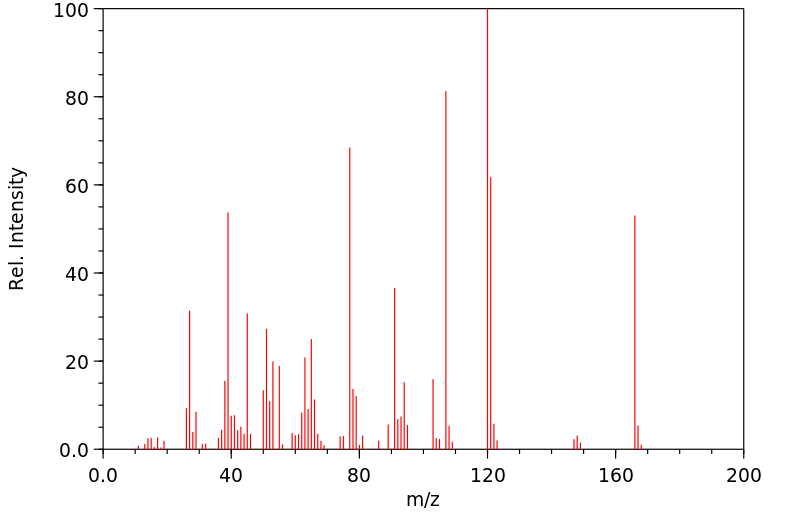
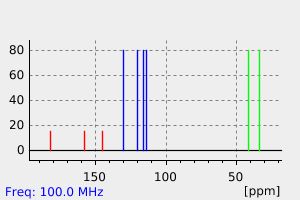
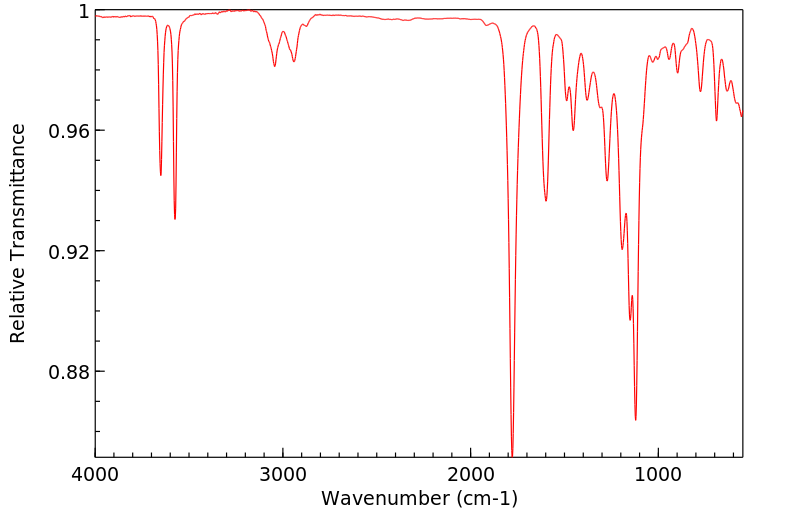
3-(3-羟基苯基)丙酸是3,4-二羟基苯基丙酸的人类已知代谢物。
3-(3-hydroxyphenyl)propanoic acid is a known human metabolite of 3,4-dihydroxyphenylpropionic acid.
来源:NORMAN Suspect List Exchange