3-(4-氯苯基)丙酸 | 2019-34-3
中文名称
3-(4-氯苯基)丙酸
中文别名
对氯苯丙酸
英文名称
3-(4-chlorophenyl)propanoic acid
英文别名
3-(4-chlorophenyl)propionic acid
CAS
2019-34-3
化学式
C9H9ClO2
mdl
MFCD00016555
分子量
184.622
InChiKey
BBSLOKZINKEUCR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:127-131 °C (lit.)
-
沸点:263.86°C (rough estimate)
-
密度:1.1989 (rough estimate)
-
稳定性/保质期:
如果按照规格正确使用和储存,则不会分解,不存在已知的危险反应,应避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:12
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.222
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xn
-
安全说明:S26,S36/37/39
-
危险类别码:R22,R41
-
WGK Germany:2
-
海关编码:2916399090
-
危险品运输编号:NONH for all modes of transport
-
危险性防范说明:P280,P305+P351+P338,P310
-
危险性描述:H302,H315,H319,H332,H335
-
储存条件:请将贮藏器密封保存,在阴凉、干燥处存放,并确保工作环境具有良好的通风或排气设施。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
3-(4-Chlorophenyl)propanoic acid
Product Name:
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H302: Harmful if swallowed
H318: Causes serious eye damage
P280: Wear protective gloves/protective clothing/eye protection/face protection
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
Section 3. Composition/information on ingredients.
Ingredient name: 3-(4-Chlorophenyl)propanoic acid
CAS number: 2019-34-3
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
This product should be handled only by, or under the close supervision of, those properly qualified
Handling:
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C9H9ClO2
Molecular weight: 184.6
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen chloride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
3-(4-Chlorophenyl)propanoic acid
Product Name:
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H302: Harmful if swallowed
H318: Causes serious eye damage
P280: Wear protective gloves/protective clothing/eye protection/face protection
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
Section 3. Composition/information on ingredients.
Ingredient name: 3-(4-Chlorophenyl)propanoic acid
CAS number: 2019-34-3
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
This product should be handled only by, or under the close supervision of, those properly qualified
Handling:
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C9H9ClO2
Molecular weight: 184.6
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen chloride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
化学性质
无色结晶。熔点为126℃(范围在121-122.5℃之间)。能溶于四氯化碳。
用途
作为除草剂麦敌散的中间体。
生产方法
由对氯苯丙烯酸电解还原制得。还原过程在装有渗透性隔膜容器的双层电解槽中进行。外槽用水银覆盖,槽底作阴极,阳极为螺旋形铅片悬于内槽中央。阴极电解液中有电动搅拌器,开始时分别向内外两槽加入7-8%的硫酸钠溶液,随后以0.5-0.7mol/L的比例将对氯肉桂酸悬浮在内槽电解液中,并启动搅拌器。在阴极电解液中按每摩尔对氯肉桂酸加入0.6-0.8mol氢氧化钠的比例添加碱溶液。接通直流电源进行电解还原,实验中的电压为10-20V,电流约为0.8-3A。还原1摩尔的对氯肉桂酸大约需要60-65A·h电量。
还原完毕后,使用倾泻法或虹吸分离阴极电解液和水银,滤除少量不溶物,并用过量硫酸(1:1)酸化处理,再经抽滤、洗涤及干燥后得到白色粉末固体,产率约为80%。通过四氯化碳提纯可获得成品。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-氯苯丙酸乙酯 ethyl 3-(4-chlorophenyl)propanoate 7116-36-1 C11H13ClO2 212.676 3-(4'-氯苯基)丙醇 3-(p-chlorophenyl)propanol 6282-88-8 C9H11ClO 170.639 3-苯基丙酸 3-Phenylpropionic acid 501-52-0 C9H10O2 150.177 [(4-氯苯基)甲基]丙二酸 <(4-chlorophenyl)methyl>propanedioic acid 21405-64-1 C10H9ClO4 228.632 3-(4-氯苯基)-丙腈 3-(4-chlorophenyl)propanenitrile 32327-71-2 C9H8ClN 165.622 3-(4-氨基苯基)丙酸 3-(4-aminophenyl)propionic acid 2393-17-1 C9H11NO2 165.192 4-氯苯丙酮 4'-chloropropiophenone 6285-05-8 C9H9ClO 168.623 2-[(4-氯苯基)甲基]丙二酸二乙酯 diethyl (4-chlorobenzyl)malonate 37556-13-1 C14H17ClO4 284.74 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 苯丙酸,4-氯-,甲酯 methyl 3-(4-chlorophenyl)propanoate 50561-69-8 C10H11ClO2 198.649 —— (R)-3-(4-chlorophenyl)-2-methylpropanoic acid 199679-74-8 C10H11ClO2 198.649 —— (S)-3-(4-chlorophenyl)-2-methylpropanoic acid 199679-72-6 C10H11ClO2 198.649 —— 3-(4-chlorophenyl)-2-methylpropanoic acid 1012-17-5 C10H11ClO2 198.649 4-氯苯丙酸乙酯 ethyl 3-(4-chlorophenyl)propanoate 7116-36-1 C11H13ClO2 212.676 3-(4-氯苯基)丙二醛 3-(4-chlorophenyl)propionaldehyde 75677-02-0 C9H9ClO 168.623 3-(4'-氯苯基)丙醇 3-(p-chlorophenyl)propanol 6282-88-8 C9H11ClO 170.639 3-苯基丙酸 3-Phenylpropionic acid 501-52-0 C9H10O2 150.177 4-(4-氯苯基)-2-丁酮 4-(4-chlorophenyl)butan-2-one 3506-75-0 C10H11ClO 182.65 —— 3-(4-chlorophenyl)-2-hydroxypropanoic acid 23434-95-9 C9H9ClO3 200.622 —— tert-butyl 3-(4-chlorophenyl)propanoate —— C13H17ClO2 240.73 —— 3-(4-chlorophenyl)propanoyl chloride 52085-96-8 C9H8Cl2O 203.068 —— 3-(4-chlorophenyl)propanamide 99839-78-8 C9H10ClNO 183.637 —— 1-(but-3-yn-1-yl)-4-chlorobenzene —— C10H9Cl 164.634 —— 1,5-bis(4-chlorophenyl)pentan-3-one 83594-12-1 C17H16Cl2O 307.219 —— 3-(4-chlorophenyl)propane-1-thiol 175219-67-7 C9H11ClS 186.705 3-(4-氯苯基)-丙胺 3-(4-chlorophenyl)propylamine 18655-50-0 C9H12ClN 169.654 1-(3-溴丙基)-4-氯苯 3-(4-chlorophenyl)propyl bromide 64473-35-4 C9H10BrCl 233.535 4-对氯苯基-1-丁烯 4-(p-chlorophenyl)-1-butene 3047-24-3 C10H11Cl 166.65 —— 3-(4-Chlorophenyl)propanoyl 2,2-dimethylpropanoate 1027659-22-8 C14H17ClO3 268.74 —— 2-chloro-3-(4-chlorophenyl)propanal 99846-92-1 C9H8Cl2O 203.068 —— methyl 3-(4-chlorophenyl)-2-hydroxypropanoate 59604-05-6 C10H11ClO3 214.649 3-(4-氯苯基)-N-甲基丙烷-1-胺 3-(4-chlorophenyl)-N-methylpropan-1-amine 90944-90-4 C10H14ClN 183.681 对羟基苯丙酸 4-hydroxyphenylpropionic acid 501-97-3 C9H10O3 166.177 1-氯-4-乙基苯 4-chloro(ethylbenzene) 622-98-0 C8H9Cl 140.612 —— 3-(4-chlorophenyl)-N-ethylpropanamide 1314661-21-6 C11H14ClNO 211.691 —— 3-(4-chlorophenyl)-N,N-dimethylpropanamide 72457-30-8 C11H14ClNO 211.691 —— 3-(2-bromo-4-chlorophenyl)propanoic acid 66192-04-9 C9H8BrClO2 263.518 —— 3-(4-chlorophenyl)propyl methanesulfonate 61440-60-6 C10H13ClO3S 248.73 —— 4-(4-chloro-phenyl)-1,1,1-trifluoro-butan-2-one —— C10H8ClF3O 236.621 4-氯苯乙醇 2-(4-Chlorophenyl)ethanol 1875-88-3 C8H9ClO 156.612 —— S-[3-(4-chlorophenyl)propyl]ethanethioate 175219-66-6 C11H13ClOS 228.743 —— 3-(4-chlorophenyl)-N-(2-methylpropyl)propanamide 940660-89-9 C13H18ClNO 239.745 —— (S)-2-Azido-3-(4-chlorophenyl)propionic acid 159750-04-6 C9H8ClN3O2 225.634 —— N-Butyl-4-chlorobenzenepropanamide 54833-21-5 C13H18ClNO 239.745 3-(4-甲氧基苯基)丙酸 3-(4-methoxyphenyl)propanoic acid 1929-29-9 C10H12O3 180.203 Pitolisant; 1-[3-[3-(4-氯苯基)丙氧基]丙基]哌啶 pitolisant 362665-56-3 C17H26ClNO 295.853 4-氯苯乙胺 4-chlorophenylethylamine 156-41-2 C8H10ClN 155.627 3-(4-氯苯基)-1-(4-甲基苯基)-1-丙酮 3-(4-chlorophenyl)-1-(p-tolyl)propan-1-one 315180-21-3 C16H15ClO 258.748 —— 3-(4-chlorophenyl)-N-cyclopropylpropanamide 1018236-91-3 C12H14ClNO 223.702 1-氯-4-(2-碘乙基)-苯 1-chloro-4-(2-iodoethyl)-benzene 57186-64-8 C8H8ClI 266.509 —— 3-(4-chlorophenyl)-N-phenethylpropanamide 26210-35-5 C17H18ClNO 287.789 —— 3-(4-chlorophenyl)-N-(cyclopropylmethyl)propanamide 1147418-36-7 C13H16ClNO 237.729 - 1
- 2
- 3
- 4
- 5
反应信息
-
作为反应物:描述:3-(4-氯苯基)丙酸 在 Zn(2,2,6,6-tetramethylpiperidine)2*2LiCl 、 bis(η3-allyl-μ-chloropalladium(II)) 、 乙酸烯丙酯 作用下, 以 四氢呋喃 为溶剂, 反应 4.5h, 以66%的产率得到对氯肉桂酸参考文献:名称:烯丙基钯催化羧酸的α,β-脱氢摘要:据报道,通过烯丙基钯催化,烯醇盐可实现高度实用且分步经济的羧酸α,β-脱氢。使用锌(TMP)时产生二价阴离子经历平滑脱氢2 ⋅2的LiCl为在过量的ZnCl的存在下与碱2,从而避免了这些基材的典型脱羧途径。直接获得2-烯酸可以通过多种方法衍生化。DOI:10.1002/anie.201706893
-
作为产物:描述:参考文献:名称:Synthesized Drug from Medicinal Plant phytochemicals Effectively Targets ECM1 Gene Mutations in Ulcerative Colitis摘要:溃疡性结肠炎(UC)是一种主要影响结肠粘膜的炎症性肠道疾病。根据其出现的方式,它可以影响整个结肠甚至远端直肠。UC可以影响男女各年龄段的人群,但大多数患者通常在15至30岁之间出现。细胞外基质蛋白-1(ECM1)基因是一个重要的候选基因,ECM1单核苷酸多态性的突变可能导致患者组织损伤,从而加剧由金属蛋白酶9引起的组织损伤,导致UC。在这项分析中,从中国齐鲁医院中医科学委员会获得了化合物合成的批准。选择了用作UC治疗的几种衍生物来构建药效团模型,采用基于配体的药效团建模方法进行虚拟筛选,以识别合适的药物化合物。然后在体外合成了选定的化合物,并使用分子对接技术进行验证。合成的化合物符合其他药物相似性法则的非毒性存在的所有特征。对接复合物中发现的特定交互氨基酸包括精氨酸(ARG):47,赖氨酸(LYS):54,苯丙氨酸(PHE):141,天冬氨酸(ASN):51,丝氨酸(SER):219,组氨酸(HIS):144,PHE:214,缬氨酸(VAL):220,酪氨酸(TYR):145和TYR:284。合成化合物与ECM1基因中突变的TYR:284的相互作用证实了药物分子作为溃疡性结肠炎治疗药物的可行性和安全性。未来可以在实验室中探索其有效性,并将这种合成化合物用作临床研究中针对ECM1基因中TYR:284突变的药物靶点。DOI:10.2174/1570180818666210804130050
文献信息
-
[EN] 2,4-DIAMINOQUINAZOLINES FOR SPINAL MUSCULAR ATROPHY<br/>[FR] 2,4-DIAMINOQUINAZOLINES UTILES POUR LE TRAITEMENT D'UNE ATROPHIE MUSCULAIRE SPINALE申请人:DECODE CHEMISTRY INC公开号:WO2005123724A1公开(公告)日:2005-12-292,4-Diaminoquinazolines of formulae I-IV and VI (I, II, III, IV and VI) are useful for treating spinal muscular atrophy (SMA).2,4-二氨基喹唑啉的化学式I-IV和VI(I,II,III,IV和VI)可用于治疗脊髓性肌萎缩症(SMA)。
-
[EN] SUBSTITUTED BENZAMIDE DERIVATIVES<br/>[FR] DÉRIVÉS DE BENZAMIDE SUBSTITUÉS申请人:HOFFMANN LA ROCHE公开号:WO2011076678A1公开(公告)日:2011-06-30The invention relates to compounds of formula I wherein R is hydrogen or lower alkyl; R1 is -(CH2)n-(O)o-heterocycloalkyl or -C(O)-heterocycloalkyl, wherein the heterocycloalkyl group is optionally substituted by lower alkyl, hydroxy, halogen or by -(CH2)p-aryl; n is 0, 1 or 2; o is 0 or 1; p is 0, 1 or 2; R2 is CF3, cycloalkyl, optionally substituted by lower alkoxy or halogen, or is indan-2-yl, or is heterocycloalkyl, optionally substituted by heteroaryl, or is aryl or heteroaryl, wherein the aromatic rings are optionally substituted by one or two substituents, selected from lower alkyl, halogen, heteroaryl, hydroxy, CF3, OCF3, OCH2CF3, OCH2-cycloalkyl, OCH2C(CH2OH)(CH2C1)(CH3), S-lower alkyl, lower alkoxy, CH2-lower alkoxy, lower alkinyl or cyano, or by-C(O)-phenyl, -O-phenyl, -O- CH2-phenyl, phenyl or -CH2-phenyl, and wherein the phenyl rings may optionally be substituted by halogen, -C(O)-lower alkyl, -C(O)OH or -C(O)O-lower alkyl, or the aromatic rings are optionally substituted by heterocycloalkyl, OCH2-oxetan-3-yl or O-tetrahydropyran-4-yl, optionally substituted by lower alkyl; X is a bond, -NR'-, -CH2NH-, -CHR''-, -(CHR'')q-O-, -O-(CHR'')q- or -(CH2)2-; Y is a bond or -CH2- R' is hydrogen or lower alkyl, R'' is hydrogen, lower alkyl, CF3, lower alkoxy, q is 0, 1, 2 or 3; or to a pharmaceutically suitable acid addition salt thereof. It has now been found that the compounds of formula I have a good affinity to the trace amine associated receptors (TAARs), especially for TAAR1. The compounds may be used for the treatment of depression, anxiety disorders, bipolar disorder, attention deficit hyperactivity disorder (ADHD), stress-related disorders, psychotic disorders such as schizophrenia, neurological diseases such as Parkinsons disease, neurodegenerative disorders such as Alzheimers disease, epilepsy, migraine, hypertension, substance abuse and metabolic disorders such as eating disorders, diabetes, diabetic complications, obesity, dyslipidemia, disorders of energy consumption and assimilation, disorders and malfunction of body temperature homeostasis, disorders of sleep and circadian rhythm, and cardiovascular disorders.该发明涉及以下式I的化合物,其中R是氢或较低的烷基;R1是-(CH2)n-(O)o-杂环烷基或-C(O)-杂环烷基,其中杂环烷基基团可选择地被较低的烷基,羟基,卤素或-( )p-芳基取代;n为0、1或2;o为0或1;p为0、1或2;R2为CF3,环烷基,可选择地被较低的烷氧基或卤素取代,或为茚-2-基,或为杂环烷基,可选择地被杂芳基取代,或为芳基或杂芳基,其中芳香环可选择地被来自较低的烷基,卤素,杂芳基,羟基, ,O ,O ,O -环烷基,O C( OH)( C1)(CH3),S-较低的烷基,较低的烷氧基, -较低的烷氧基,较低的炔基或氰基,或-C(O)-苯基,-O-苯基,-O- -苯基,苯基或- -苯基选择的一个或两个取代基取代,其中苯环可选择地被卤素,-C(O)-较低的烷基,-C(O)OH或-C(O)O-较低的烷基取代,或芳香环可选择地被杂环烷基,O -氧杂环戊烷-3-基或O-四氢吡喃-4-基,可选择地被较低的烷基取代;X为键,-NR'-,- NH-,-CHR''-,-(CHR'')q-O-,-O-(CHR'')q-或-( )2-;Y为键或- -;R'为氢或较低的烷基,R''为氢,较低的烷基, ,较低的烷氧基,q为0、1、2或3;或其药学上适宜的酸盐。现已发现,该式I的化合物对痕量胺相关受体(TAARs)具有良好的亲和力,特别是对于TAAR1。这些化合物可用于治疗抑郁症,焦虑症,躁郁症,注意力缺陷多动障碍(ADHD),与压力有关的疾病,如精神分裂症,帕金森病等神经疾病,阿尔茨海默病等神经退行性疾病,癫痫,偏头痛,高血压,物质滥用和代谢性疾病,如进食障碍,糖尿病,糖尿病并发症,肥胖症,血脂异常,能量消耗和吸收异常,体温稳态异常,睡眠和昼夜节律异常,以及心血管疾病。
-
Pentafluorophenyl Esters: Highly Chemoselective Ketyl Precursors for the Synthesis of α,α-Dideuterio Alcohols Using SmI<sub>2</sub> and D<sub>2</sub>O as a Deuterium Source作者:Hengzhao Li、Yuxia Hou、Chengwei Liu、Zemin Lai、Lei Ning、Roman Szostak、Michal Szostak、Jie AnDOI:10.1021/acs.orglett.9b04383日期:2020.2.21We report the first highly chemoselective synthesis of α,α-dideuterio alcohols with exquisite incorporation of deuterium (>98% [D2]) using pentafluorophenyl esters as ketyl radical precursors, SmI2 as a mild reducing agent, and D2O as the deuterium source. This system tolerates a variety of functional groups, offering rapid entry to valuable α,α-dideuterated alcohol building blocks. More generally
-
Palladium-Catalyzed Reduction of Acid Chlorides to Aldehydes with Hydrosilanes作者:Yasushi Tsuji、Tetsuaki Fujihara、Cong Cong、Tomohiro Iwai、Jun TeraoDOI:10.1055/s-0032-1317155日期:——efficient synthesis of aldehydes from acid chlorides with hydrosilanes as a reducing agent in the presence of a palladium catalyst has been achieved. A simple mixture of commercially available Pd(dba)2 and Mes3P as a catalyst realized the reduction of various acid chlorides including aliphatic acid chlorides and α,β-unsaturated acid chlorides to the corresponding aldehydes in good to high yields under
-
Ligand-controlled divergent dehydrogenative reactions of carboxylic acids via C–H activation作者:Zhen Wang、Liang Hu、Nikita Chekshin、Zhe Zhuang、Shaoqun Qian、Jennifer X. Qiao、Jin-Quan YuDOI:10.1126/science.abl3939日期:2021.12.3inaccessible with existing carbonyl desaturation protocols. Product inhibition is overcome through ligand-promoted preferential activation of C(sp3)–H bonds over C(sp2)–H bonds or a tandem dehydrogenation or vinyl C–H alkynylation sequence. The dehydrogenation reaction is compatible with molecular oxygen as the terminal oxidant.
表征谱图
-
氢谱1HNMR
-
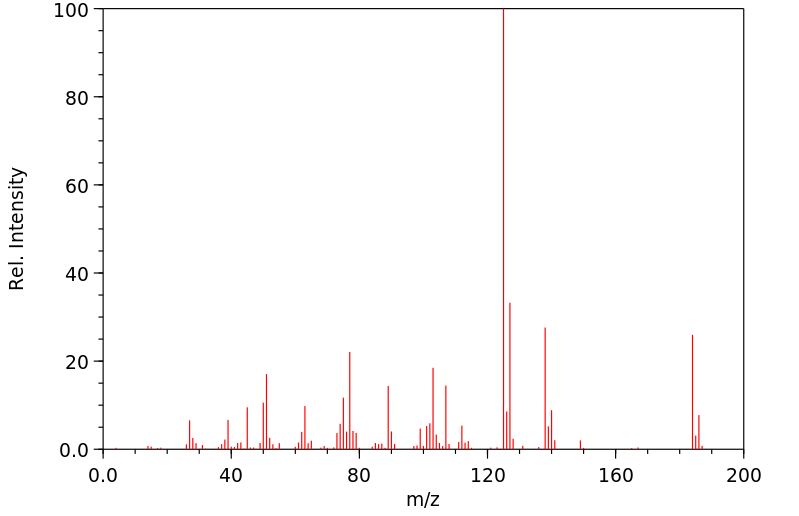
质谱MS
-
碳谱13CNMR
-
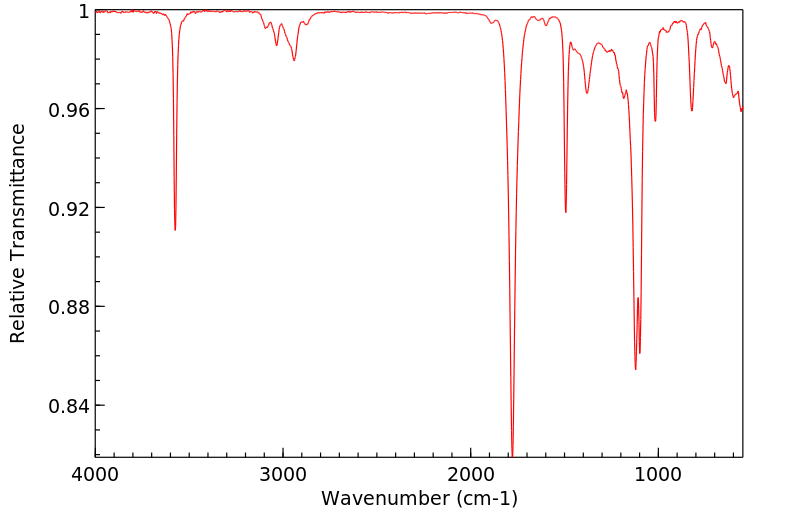
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
限制性核酸内切酶TAQⅠ(TTHHB8I)
阿明洛芬
阿拉洛芬
铁-N-(3-苯基戊二酰)去铁敏B
钙二[(2R)-2-羟基-3-苯丙酸酯]
酮洛芬相关物质C
酪泮酸钠
酪氨酸,3-羟基-b-亚甲基-
苯基丙酮酸缩氨基脲
苯基丁二酸
苯乙酸,a-甲基-4-(4,5,6,7-四氢-2-苯并噻唑基)-
苯丙酸钠盐
苯丙酸钙盐(2:1)
苯丙酸,加合N-环己基并环己胺(1:1)
苯丙酸,b-[[(苯基氨基)羰基]氨基]-
苯丙酸,b-[[(二乙胺基)硫代甲基]硫代]-
苯丙酸,a-[2-[甲基[2-(4-吗啉基)乙基]氨基]-2-羰基乙基]-,(R)-
苯丙酸,a-[(乙酰基硫代)甲基]-,(S)-
苯丙酸,4-羟基-b,2,6-三甲基-,(bR)-
苯丙酸,4-氯-a-(肟基)-
苯丙酸,3-硝基-b-(三氯甲锗烷基)-
苯丙酸,3-氯-a-羟基-
苯丙酸 羟基-4-甲氧基
苄氧羰基-DL-beta-苯丙氨酸
苄基马来酸
苄基丙二酸单酰肼
苄基丙二酸
苄基丁酸
艾司洛尔酸钠
艾司洛尔酸
胆影脒
羧基布洛芬
羟基布洛芬
美索洛芬
米格列奈
米格列奈
碘芬酸
碘番酸
碘泊酸钠
碘泊酸钙
硬脂酰胺丙基鲸蜡硬脂基二甲基铵甲苯磺酸盐
番石榴酸
甲酪氨酸
甲基多巴杂质A
甲基多巴EP杂质B
甲基多巴
甲基3-(4-苄氧基-2-甲基-苯基)丙酸酯
消旋甲酪氨酸
消旋布洛芬赖氨酸盐
消旋卡多曲二元酸杂质