5,5,5',5'-tetramethyl-2,2'-ethylidene-bis-cyclohexane-1,3-dione | 3316-11-8
中文名称
——
中文别名
——
英文名称
5,5,5',5'-tetramethyl-2,2'-ethylidene-bis-cyclohexane-1,3-dione
英文别名
2,2'-ethylidenebis(5,5-dimethylcyclohexane-1,3-dione);1,1-Bis(4,4-dimethyl-2,6-dioxohexyl)ethane;5,5,5',5'-Tetramethyl-2,2'-aethyliden-bis-cyclohexan-1,3-dion;1.1-Bis-(2.6-dioxo-4.4-dimethyl-cyclohexyl)-aethan;Aethyliden-bis-dimethyl-dihydroresorcin;1,1-Bis(4,4-dimethyl-2,6-dioxocyclohexyl)ethane;2-[1-(4,4-dimethyl-2,6-dioxocyclohexyl)ethyl]-5,5-dimethylcyclohexane-1,3-dione
CAS
3316-11-8
化学式
C18H26O4
mdl
——
分子量
306.402
InChiKey
FCDITYROBVWZOO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:459.4±40.0 °C(Predicted)
-
密度:1.079±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:22
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.78
-
拓扑面积:68.3
-
氢给体数:0
-
氢受体数:4
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 5,5-二甲基-1,3-环己二酮 dimedone 126-81-8 C8H12O2 140.182
反应信息
-
作为反应物:描述:5,5,5',5'-tetramethyl-2,2'-ethylidene-bis-cyclohexane-1,3-dione 在 乙酸铵 作用下, 以 溶剂黄146 为溶剂, 反应 2.0h, 以92%的产率得到3,3,6,6,9-pentamethyl-1,2,3,4,5,6,7,8,9,10-decahydroacridine-1,8-dione参考文献:名称:Synthesis, Characterization, and Electrochemistry of Some Acridine-1,8-dione Dyes摘要:The synthesis, characterization, and electrochemical behavior of some acridinedione derivatives are reported. Cyclic voltammetric studies show that all the dyes undergo irreversible oxidation irrespective of the substitution on the nitrogen. The product formed on oxidation is the aromatic derivative in the case of N-H compounds and the acridinium salt in the case of the N-substituted compounds, which have been isolated and characterized. Formation of an intermediate carbon-centered radical is observed as evidenced by ESR spin-trapping experiments. A mechanistic scheme for the electrochemical oxidation is proposed. On carrying out reduction after oxidation, different products are formed depending on the substitution on the nitrogen. There is no reduction of the oxidized product in the case of N-H compounds, and compounds with substitution on nitrogen undergo reduction consistent with the observation in N-alkylpyridinium salts.DOI:10.1021/jo9600316
-
作为产物:参考文献:名称:Eistert,B. et al., Chemische Berichte, 1968, vol. 101, p. 84 - 93摘要:DOI:
文献信息
-
Merging supramolecular catalysis and aminocatalysis: amino-appended β-cyclodextrins (ACDs) as efficient and recyclable supramolecular catalysts for the synthesis of tetraketones作者:Yufeng Ren、Bo Yang、Xiali LiaoDOI:10.1039/c6ra01002d日期:——β-CD were synthesized and employed in the catalytic synthesis of a series of tetraketones as supramolecular catalysts in water for the first time. Yields of 58–97% were obtained with up to 30 examples of substrate. The catalyst could be recycled easily, while a 92% yield and 84% rate of catalyst recovery could be achieved after 8 cycles of catalyst recycling. Moreover, a catalytic mechanism merging
-
Electrophilic substitution in pyrroles. Part 5. Reaction of dipyrrylmethanes with arenediazonium ions作者:Anthony R. Butler、Peter T. ShepherdDOI:10.1039/p29800000113日期:——Dipyrrylmethanes react with arenediazonium ions in acid solution to form azopyrroles and formaldehyde. At low pH cleavage of the methylene bridge is brought about by attack of hydrogen ions, but at higher pH arenediazonium ions assume this role. In the reaction of benzylpyrrole, benzyl alcohol was detected as a product, suggesting formation of a benzyl carbonium ion. The relevance of these studies to the
-
Vorlaender, Fresenius Zeitschrift fur Analytische Chemie, 1929, vol. 77, p. 266作者:VorlaenderDOI:——日期:——
-
Lapkin,I.I.; Kashinskii,V.N., Journal of Organic Chemistry USSR (English Translation), 1973, vol. 9, p. 309 - 311作者:Lapkin,I.I.、Kashinskii,V.N.DOI:——日期:——
-
METHONE DERIVATIVES OF ALDEHYDES作者:E. C. HORNING、M. G. HORNINGDOI:10.1021/jo01171a014日期:1946.1
表征谱图
-
氢谱1HNMR
-
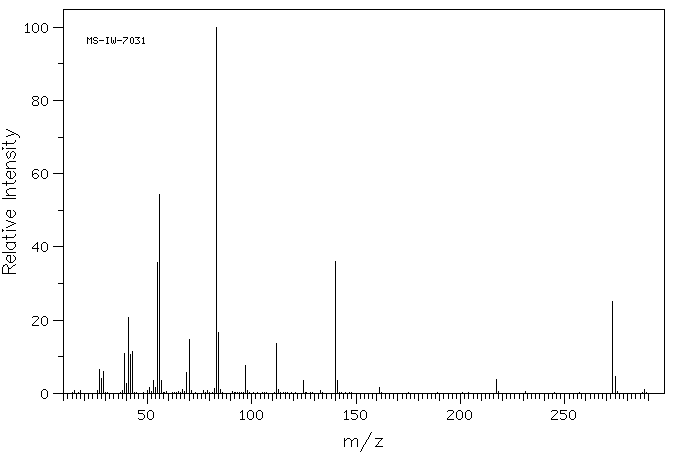
质谱MS
-
碳谱13CNMR
-
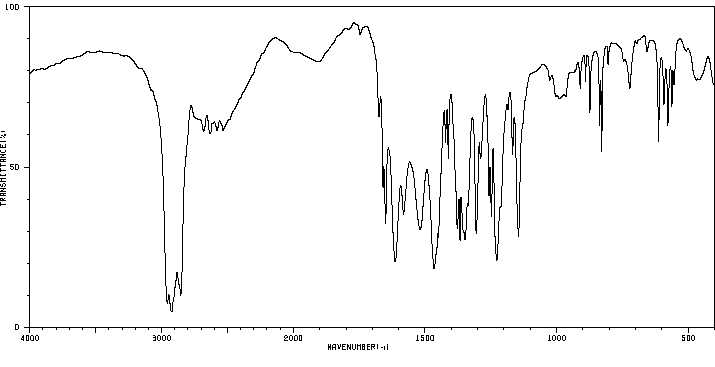
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β,6α,8α,10α,13α)-6-羟基-15-氧代黄-9(11),16-二烯-18-油酸
(3S,3aR,8aR)-3,8a-二羟基-5-异丙基-3,8-二甲基-2,3,3a,4,5,8a-六氢-1H-天青-6-酮
(2Z)-2-(羟甲基)丁-2-烯酸乙酯
(2S,4aR,6aR,7R,9S,10aS,10bR)-甲基9-(苯甲酰氧基)-2-(呋喃-3-基)-十二烷基-6a,10b-二甲基-4,10-dioxo-1H-苯并[f]异亚甲基-7-羧酸盐
(1aR,4E,7aS,8R,10aS,10bS)-8-[((二甲基氨基)甲基]-2,3,6,7,7a,8,10a,10b-八氢-1a,5-二甲基-氧杂壬酸[9,10]环癸[1,2-b]呋喃-9(1aH)-酮
(+)顺式,反式-脱落酸-d6
龙舌兰皂苷乙酯
龙脑香醇酮
龙脑烯醛
龙脑7-O-[Β-D-呋喃芹菜糖基-(1→6)]-Β-D-吡喃葡萄糖苷
龙牙楤木皂甙VII
龙吉甙元
齿孔醇
齐墩果醛
齐墩果酸苄酯
齐墩果酸甲酯
齐墩果酸溴乙酯
齐墩果酸二甲胺基乙酯
齐墩果酸乙酯
齐墩果酸3-O-alpha-L-吡喃鼠李糖基(1-3)-beta-D-吡喃木糖基(1-3)-alpha-L-吡喃鼠李糖基(1-2)-alpha-L-阿拉伯糖吡喃糖苷
齐墩果酸 beta-D-葡萄糖酯
齐墩果酸 beta-D-吡喃葡萄糖基酯
齐墩果酸 3-乙酸酯
齐墩果酸 3-O-beta-D-葡吡喃糖基 (1→2)-alpha-L-吡喃阿拉伯糖苷
齐墩果酸
齐墩果-12-烯-3b,6b-二醇
齐墩果-12-烯-3,24-二醇
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,11-二酮
齐墩果-12-烯-2α,3β,28-三醇
齐墩果-12-烯-29-酸,3,22-二羟基-11-羰基-,g-内酯,(3b,20b,22b)-
齐墩果-12-烯-28-酸,3-[(6-脱氧-4-O-b-D-吡喃木糖基-a-L-吡喃鼠李糖基)氧代]-,(3b)-(9CI)
齐墩果-12-烯-28-酸,3,7-二羰基-(9CI)
齐墩果-12-烯-28-酸,3,21,29-三羟基-,g-内酯,(3b,20b,21b)-(9CI)
鼠特灵
鼠尾草酸醌
鼠尾草酸
鼠尾草酚酮
鼠尾草苦内脂
黑蚁素
黑蔓醇酯B
黑蔓醇酯A
黑蔓酮酯D
黑海常春藤皂苷A1
黑檀醇
黑果茜草萜 B
黑五味子酸
黏黴酮
黏帚霉酸