盐酸胡芦巴碱 | 6138-41-6
中文名称
盐酸胡芦巴碱
中文别名
N-甲基烟酸内盐盐酸盐;胡芦巴碱盐酸盐;盐酸葫芦巴碱葫芦巴碱盐酸盐、N-甲基烟酸内盐盐酸盐;盐酸葫芦巴碱;葫芦巴碱盐酸盐;胡卢巴碱盐酸盐
英文名称
trigonelline hydrochloride
英文别名
trigonelline chloride;trigonelline;1-Methylpyridinium-3-carboxylate hydrochloride;1-methylpyridin-1-ium-3-carboxylate;hydrochloride
CAS
6138-41-6
化学式
C7H8NO2*Cl
mdl
MFCD00079597
分子量
173.599
InChiKey
TZSYLWAXZMNUJB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:~260 °C (dec.)
-
溶解度:少许溶于甲醇、可溶于水
-
碰撞截面:124.1 Ų [M+H]+; 135.9 Ų [M+Na]+
计算性质
-
辛醇/水分配系数(LogP):-3.53
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:41.2
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险品标志:Xi
-
安全说明:S22,S24/25,S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2933399090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:-20°C
SDS
SECTION 1: Identification of the substance/mixture and of the company/undertaking
Product identifiers
Product name : Trigonelline hydrochloride
REACH No. : A registration number is not available for this substance as the substance
or its uses are exempted from registration, the annual tonnage does not
require a registration or the registration is envisaged for a later
registration deadline.
CAS-No. : 6138-41-6
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
SECTION 2: Hazards identification
Classification of the substance or mixture
Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008.
This substance is not classified as dangerous according to Directive 67/548/EEC.
Label elements
The product does not need to be labelled in accordance with EC directives or respective national laws.
Other hazards - none
SECTION 3: Composition/information on ingredients
Substances
Synonyms : 1-Methylpyridinium-3-carboxylatehydrochloride
N-Methylnicotinic acid betainehydrochloride
Formula : C7H7NO2 · HCl
Molecular Weight : 173,60 g/mol
CAS-No. : 6138-41-6
EC-No. : 228-119-5
No components need to be disclosed according to the applicable regulations.
SECTION 4: First aid measures
Description of first aid measures
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration.
In case of skin contact
Wash off with soap and plenty of water.
In case of eye contact
Flush eyes with water as a precaution.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water.
Most important symptoms and effects, both acute and delayed
The most important known symptoms and effects are described in the labelling (see section 2.2) and/or in
section 11
Indication of any immediate medical attention and special treatment needed
no data available
SECTION 5: Firefighting measures
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides, nitrogen oxides (NOx), Hydrogen chloride gas
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
SECTION 6: Accidental release measures
Personal precautions, protective equipment and emergency procedures
Avoid dust formation. Avoid breathing vapours, mist or gas.
For personal protection see section 8.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Sweep up and shovel. Keep in suitable, closed containers for disposal.
Reference to other sections
For disposal see section 13.
SECTION 7: Handling and storage
Precautions for safe handling
Provide appropriate exhaust ventilation at places where dust is formed.Normal measures for preventive fire
protection.
For precautions see section 2.2.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Specific end use(s)
Apart from the uses mentioned in section 1.2 no other specific uses are stipulated
SECTION 8: Exposure controls/personal protection
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
General industrial hygiene practice.
Personal protective equipment
Eye/face protection
Use equipment for eye protection tested and approved under appropriate government standards
such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
Choose body protection in relation to its type, to the concentration and amount of dangerous
substances, and to the specific work-place., The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Respiratory protection is not required. Where protection from nuisance levels of dusts are desired,
use type N95 (US) or type P1 (EN 143) dust masks. Use respirators and components tested and
approved under appropriate government standards such as NIOSH (US) or CEN (EU).
Control of environmental exposure
Do not let product enter drains.
SECTION 9: Physical and chemical properties
Information on basic physical and chemical properties
a) Appearance Form: crystalline
Colour: white
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing Melting point/range: 260 °C
point
f) Initial boiling point and no data available
boiling range
g) Flash point no data available
h) Evapouration rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility no data available
o) Partition coefficient: n- no data available
octanol/water
p) Auto-ignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
SECTION 10: Stability and reactivity
Reactivity
no data available
Chemical stability
Stable under recommended storage conditions.
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
In the event of fire: see section 5
SECTION 11: Toxicological information
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitisation
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Additional Information
RTECS: Not available
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
SECTION 12: Ecological information
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
PBT/vPvB assessment not available as chemical safety assessment not required/not conducted
Other adverse effects
no data available
SECTION 13: Disposal considerations
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company.
Contaminated packaging
Dispose of as unused product.
SECTION 14: Transport information
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
SECTION 15: Regulatory information
This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.
Safety, health and environmental regulations/legislation specific for the substance or mixture
no data available
Chemical Safety Assessment
For this product a chemical safety assessment was not carried out
SECTION 16: Other information
Further information
Copyright 2014 Co. LLC. License granted to make unlimited paper copies for internal use
only.
The above information is believed to be correct but does not purport to be all inclusive and shall be
used only as a guide. The information in this document is based on the present state of our knowledge
and is applicable to the product with regard to appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Corporation and its Affiliates shall not be held
liable for any damage resulting from handling or from contact with the above product. See
and/or the reverse side of invoice or packing slip for additional terms and conditions of sale.
制备方法与用途
生物活性
Trigonelline氯化物是一种大量存在于咖啡中的生物碱,并且具有潜在的抗糖尿病活性。此外,它还表现出对HSV-1、抗菌和抗真菌的活性。
体外研究研究表明,Trigonelline氯化物(TG)显著改善了H9c2细胞的形态。该化合物能够减轻过氧化氢(H₂O₂)诱导的细胞死亡并提高抗氧化作用。此外,在H₂O₂诱导的氧化应激条件下,Trigonelline氯化物调节了凋亡基因caspase-3、caspase-9和抗凋亡基因Bcl-2、Bcl-XL。流式细胞术结果进一步证实,Trigonelline氯化物显著减少了H₂O₂诱导的H9c2细胞坏死和凋亡。然而,随着Trigonelline氯化物浓度的增加,它也可能加剧了H₂O₂对细胞的影响。
体内研究在链脲佐星诱导的糖尿病大鼠中,Trigonelline氯化物降低了骨矿化并倾向于恶化骨骼机械性能。而在烟酰胺/链脲佐星处理的大鼠中,Trigonelline氯化物显著增加了骨密度(BMD),并有利于改善松质骨强度。因此,在链脲佐星诱导的代谢紊乱大鼠的骨骼系统中,Trigonelline氯化物表现出不同的影响,加剧了链脲佐星治疗大鼠中的骨质疏松性变化,并对非高血糖症(烟酰胺/链脲佐星处理)大鼠的骨骼产生了有利的影响。
化学性质 用途反应信息
-
作为反应物:描述:参考文献:名称:The Quasi-Favorskii Rearrangement. I. The Preparation of Demerol and β-Pethidine摘要:DOI:10.1021/ja01514a047
-
作为产物:描述:参考文献:名称:高浓度水溶液中三种相关双(吡啶)醛肟的降解:意外快速酰胺基水解的例子摘要:发现在浓度接近潜在治疗剂量浓度的浓非缓冲水溶液中,两种双(吡啶)醛肟肟有机磷酸酯抑制的乙酰胆碱酯酶活化剂1(HI-6)和3(HS-6)的主要初始降解产物是羧酸衍生物。由酰胺官能团的水解形成的图2和4。通过在高浓度羟胺盐酸盐存在下加热1和3制备化合物2和4,并通过1H和13C NMR,IR和UV分析对其进行表征。确定了在类似条件下1和3以及模型化合物5、7和8中酰胺基水解速率的估算值。DOI:10.1002/jps.2600820805
文献信息
-
One-dimensional Strands of Trigonellinium Constructed by Intermolecular Strong O–H···O and Weak C–H···O Hydrogen Bonds in the Crystal of Partially Oxidized Ni(dmit)<sub>2</sub>Salt作者:Kazuaki Tomono、Kazuo MiyamuraDOI:10.1246/cl.2007.1466日期:2007.12.5The trigonellinium cations in the partially oxidized Ni(dmit)2 complex salt 1 formed strong O–H···O hydrogen-bridged dimers, and the dimers are held together by the weak C(sp2 and sp3)–H···O hydrogen bond to form 1D strands. Further assembly through weak C(sp3)–H···O hydrogen bond and π···π interaction resulted in the formation of Ni(dmit)2 column structure.部分氧化的 Ni(dmit)2 复盐 1 中的三角鎓阳离子形成了强 O-H-O 氢桥二聚体,二聚体通过弱 C(sp2 和 sp3)-H-O 氢键结合在一起形成一维链。通过弱C(sp3)-H---O氢键和π---π相互作用进一步组装,形成了Ni(dmit)2柱状结构。
-
Substituent Effects on Formation of Cation Dimers by Weak Hydrogen Bonds in Crystals of Carbonyl Pyridinium Salts of Ni(dmit)<sub>2</sub>作者:Kazuaki Tomono、Ayako Koyano、Takashi Morita、Kazuo MiyamuraDOI:10.1246/bcsj.82.1152日期:2009.9.15Five 1:1 salts of 3-X-1-methylpyridinium (X = benzoyl, acetyl, methoxycarbonyl, carboxy, and aminocarbonyl; abbreviated as Ben, Ace, Met, Car, and Ami, respectively) cations with a [Ni(dmit)2]− anion ([Ben]+[Ni(dmit)2]− (1), [Ace]+[Ni(dmit)2]− (2), [Met]+[Ni(dmit)2]− (3), [Car]+[Ni(dmit)2]− (4), and [Ami]+[Ni(dmit)2]− (5)) have been synthesized and characterized by single-crystal X-ray analysis and conductivity measurements. In the crystals, three cations 1–3 were found to form dimers by weak C–H···O hydrogen bonds, and the arrangements of cations had a strong relation with the electronic effect of the substituents. The cations of 1–3 formed similar centrosymmetrically associated dimers constructed by weak C–H···O hydrogen bonds, whose geometric parameters had a correlation with the electronic effect of the substituents. A cation of 4 also formed centrosymmetrically associated dimer, but it was made by an O–H···O hydrogen bond as usually observed in the case of carboxylic acid. In contrast with other complex salts, cations of 5 formed one-dimensional structure by C–H···O hydrogen bonding. The conductivities of salts 1, 2, 3, 4, and 5 at room temperature were 1.00 × 10−6, 1.10 × 10−6, 2.86 × 10−6, 9.77 × 10−6, and 8.75 × 10−7 S cm−1, respectively.五种 1:3-X-1-甲基吡啶鎓(X = 苯甲酰基、乙酰基、甲氧羰基、羧基和氨基羰基;缩写分别为 Ben、Ace、Met、Car 和 Ami)阳离子与[Ni(dmit)2]-阴离子([Ben]+[Ni(dmit)2]- (1)、[Ace]+[Ni(dmit)2]- (2)、[Met]+[Ni(dmit)2]- (3)、合成了[Car]+[Ni(dmit)2]- (4) 和 [Ami]+[Ni(dmit)2]- (5)),并通过单晶 X 射线分析和电导率测量进行了表征。在晶体中发现,1-3 三个阳离子通过弱 C-H-O 氢键形成二聚体,阳离子的排列与取代基的电子效应有很大关系。1-3 的阳离子通过弱 C-H-O 氢键形成了类似的中心对称关联二聚体,其几何参数与取代基的电子效应有关。4 的阳离子也形成了中心对称关联的二聚体,但它是通过 O-H-O 氢键形成的,就像通常在羧酸中观察到的那样。与其他复合盐相反,5 的阳离子通过 C-H-O 氢键形成一维结构。盐 1、2、3、4 和 5 在室温下的电导率分别为 1.00 × 10-6、1.10 × 10-6、2.86 × 10-6、9.77 × 10-6 和 8.75 × 10-7 S cm-1。
-
Case to case study for exploring inclusion complexes of an anti-diabetic alkaloid with α and β cyclodextrin molecules for sustained dischargement作者:Raja Ghosh、Kanak Roy、Arunika Subba、Palash Mandal、Sankar Basak、Mitali Kundu、Mahendra Nath RoyDOI:10.1016/j.molstruc.2019.126988日期:2020.1Abstract The host-guest inclusion complex between an anti-diabetic alkaloid and either α or β-Cyclodextrin was studied by conductivity, surface tension, UV–vis, FTIR, NMR, and Mass spectroscopy. Cyclodextrin-alkaloid inclusion complex (IC) was found to exhibit a distinct change in the surface morphology, crystalline nature & size in TEM. ICs modified nature was further explored by powder XRD, DLS.
-
PHARMACEUTICAL COMPOSITION AND A PROCESS THEREOF申请人:Bhaskaran Sunil公开号:US20080221173A1公开(公告)日:2008-09-11The present invention relates to a pharmaceutical composition having dopaminergic activity and other related pharmaceutical activities comprising trigonelline or its derivative(s) and 4-hydroxyisoleucine or its derivative(s), optionally along with excipients(s); a process of preparing a pharmaceutical composition comprising trigonelline or its derivative(s) and 4-hydroxyisoleucine or its derivative(s), optionally along with excipients(s), wherein the process comprising steps of: (a) extracting a clear solution containing trigonelline and 4-hydroxyisoleucine from plant source; and (b) optionally precipitating derivative(s) of trigonelline and 4-hydroxyisoleucine from the clear solution and obtaining said composition; and an in-vitro method to increase levels of dopamine or to inhibit prolactin by allowing composition comprising trigonelline or its derivative(s) and 4-hydroxyisoleucine or its derivative(s) to bind to cell receptors.
-
Alkylpyridiniums. 1. Formation in Model Systems via Thermal Degradation of Trigonelline作者:Richard H. Stadler、Natalia Varga、Jörg Hau、Francia Arce Vera、Dieter H. WeltiDOI:10.1021/jf011234k日期:2002.2.1Trigonelline is a well-known precursor of flavor/aroma compounds in coffee and undergoes significant degradation during roasting. This study investigates the major nonvolatile products that are procured after trigonelline has been subjected to mild pyrolysis conditions (220-250 degrees C) under atmospheric pressure. Various salt forms of trigonelline were also prepared and the thermally produced nonvolatilesTrigonelline是咖啡中香精/香气化合物的众所周知的前体,在烘焙过程中会发生明显降解。这项研究调查了将Trigonelline在大气压力下经过温和的热解条件(220-250摄氏度)后购买的主要非挥发性产品。还制备了各种形式的藜芦啉盐,并通过薄层色谱,液相色谱-电喷雾电离串联质谱,(1)H和(13)C核磁共振分析了热产生的非挥发性物质。结果显示,脱羧衍生物1-甲基吡啶鎓是某些盐的主要产物,其形成与温度从220到245摄氏度呈正相关。与一水合物相比,在所研究的温度范围内,盐酸三氯萘林可提供大量的1-甲基吡啶鎓。对trigonelline的其他潜在的季胺产物的研究也表明,导致亲核取代反应会导致二烷基吡啶鎓,尽管其浓度水平比1-甲基吡啶鎓所记录的浓度低约100倍。
表征谱图
-
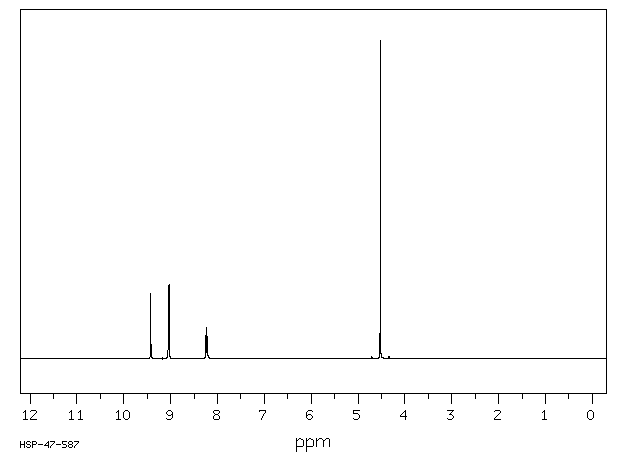
氢谱1HNMR
-
质谱MS
-
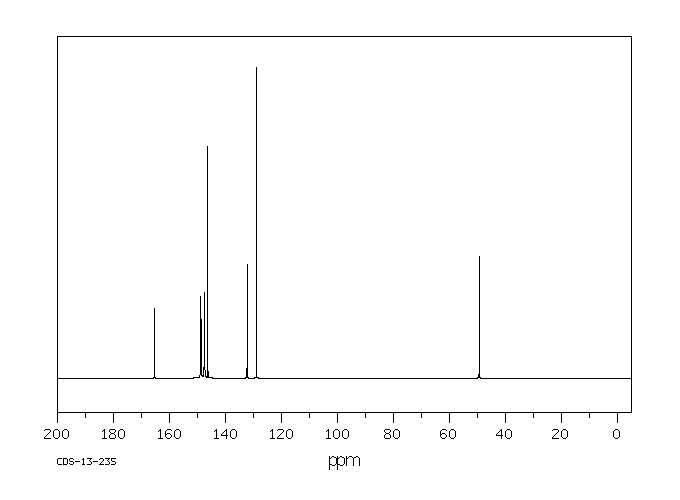
碳谱13CNMR
-
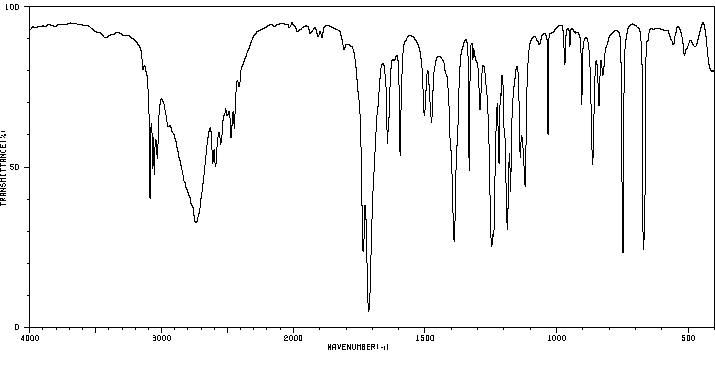
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-氨氯地平-d4
(R,S)-可替宁N-氧化物-甲基-d3
(R)-(+)-2,2'',6,6''-四甲氧基-4,4''-双(二苯基膦基)-3,3''-联吡啶(1,5-环辛二烯)铑(I)四氟硼酸盐
(R)-N'-亚硝基尼古丁
(R)-DRF053二盐酸盐
(5E)-5-[(2,5-二甲基-1-吡啶-3-基-吡咯-3-基)亚甲基]-2-亚磺酰基-1,3-噻唑烷-4-酮
(5-溴-3-吡啶基)[4-(1-吡咯烷基)-1-哌啶基]甲酮
(5-氨基-6-氰基-7-甲基[1,2]噻唑并[4,5-b]吡啶-3-甲酰胺)
(2S,2'S)-(-)-[N,N'-双(2-吡啶基甲基]-2,2'-联吡咯烷双(乙腈)铁(II)六氟锑酸盐
(2S)-2-[[[9-丙-2-基-6-[(4-吡啶-2-基苯基)甲基氨基]嘌呤-2-基]氨基]丁-1-醇
(2R,2''R)-(+)-[N,N''-双(2-吡啶基甲基)]-2,2''-联吡咯烷四盐酸盐
(1'R,2'S)-尼古丁1,1'-Di-N-氧化物
黄色素-37
麦斯明-D4
麦司明
麝香吡啶
鲁非罗尼
鲁卡他胺
高氯酸N-甲基甲基吡啶正离子
高氯酸,吡啶
高奎宁酸
马来酸溴苯那敏
马来酸氯苯那敏-D6
马来酸左氨氯地平
顺式-双(异硫氰基)(2,2'-联吡啶基-4,4'-二羧基)(4,4'-二-壬基-2'-联吡啶基)钌(II)
顺式-二氯二(4-氯吡啶)铂
顺式-二(2,2'-联吡啶)二氯铬氯化物
顺式-1-(4-甲氧基苄基)-3-羟基-5-(3-吡啶)-2-吡咯烷酮
顺-双(2,2-二吡啶)二氯化钌(II) 水合物
顺-双(2,2'-二吡啶基)二氯化钌(II)二水合物
顺-二氯二(吡啶)铂(II)
顺-二(2,2'-联吡啶)二氯化钌(II)二水合物
韦德伊斯试剂
非那吡啶
非洛地平杂质C
非洛地平
非戈替尼
非布索坦杂质66
非尼拉朵
非尼拉敏
雷索替丁
阿雷地平
阿瑞洛莫
阿扎那韦中间体
阿培利司N-6
阿伐曲波帕杂质40
间硝苯地平
间-硝苯地平
镉,二碘四(4-甲基吡啶)-
锌,二溴二[4-吡啶羧硫代酸(2-吡啶基亚甲基)酰肼]-