4,4'-二甲氧基苯酚酯 | 1226-42-2
中文名称
4,4'-二甲氧基苯酚酯
中文别名
茴香偶酰;4,4'-二甲氧基苯偶酰;4,4’-二甲氧基苯酚酯;4,4′-二甲氧基苯偶酰;4,4"-二甲氧基苯酚酯;聯大茴香醯;4,4"-二甲氧基苯偶酰
英文名称
1,2-bis(4-methoxyphenyl)-1,2-ethanedione
英文别名
4,4'-dimethoxybenzil;1,2-bis(4-methoxyphenyl)ethane-1,2-dione;p-anisil;4,4′-dimethoxy-benzil;4,4’-dimethoxybenzil;anisil
CAS
1226-42-2
化学式
C16H14O4
mdl
MFCD00008405
分子量
270.285
InChiKey
YNANGXWUZWWFKX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:132-134 °C(lit.)
-
沸点:373.4°C (rough estimate)
-
密度:1.2117 (rough estimate)
-
溶解度:DMSO:50 mg/mL (184.99 mM);水:< 0.1 mg/mL(不溶)
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,不存在已知的危险反应。应避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:20
-
可旋转键数:5
-
环数:2.0
-
sp3杂化的碳原子比例:0.125
-
拓扑面积:52.6
-
氢给体数:0
-
氢受体数:4
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S22,S24/25,S26,S36/37
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2914509090
-
危险品运输编号:NONH for all modes of transport
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:请将贮藏器保持密封,并存放在阴凉干燥处。同时,确保工作环境具有良好的通风或排气设施。
SDS
1.1 产品标识符
: 4,4′-Dimethoxybenzil
化学品俗名或商品名
1.2 鉴别的其他方法
Di-p-anisoyl
p-Anisil
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
根据全球协调系统(GHS)的规定,不是危险物质或混合物。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: Di-p-anisoyl
别名
p-Anisil
: C16H14O4
分子式
: 270.28 g/mol
分子量
成分 浓度
4,4'-Dimethoxybenzil
-
化学文摘编号(CAS No.) 1226-42-2
EC-编号 214-960-5
模块 4. 急救措施
4.1 必要的急救措施描述
如果吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。
在皮肤接触的情况下
用肥皂和大量的水冲洗。
在眼睛接触的情况下
用水冲洗眼睛作为预防措施。
如果误服
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。
4.2 最重要的症状和影响,急性的和滞后的
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 救火人员的预防
如必要的话,戴自给式呼吸器去救火。
5.4 进一步的信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
防止粉尘的生成。 防止吸入蒸汽、气雾或气体。
6.2 环境预防措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
扫掉和铲掉。 存放在合适的封闭的处理容器内。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制/个体防护
8.1 控制参数
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
人身保护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所来选择人体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 结晶
颜色: 黄色
b) 气味
无数据资料
c) 气味临界值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/熔点范围: 132 - 134 °C - lit.
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 可燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 相对蒸气密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) 辛醇/水分配系数的对数值
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 化学稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 避免接触的条件
无数据资料
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤腐蚀/刺激
无数据资料
严重眼损伤 / 眼刺激
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞诱变
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 生物积累的潜在可能性
无数据资料
12.4 土壤中的迁移
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
污染了的包装物
作为未用过的产品弃置。
模块 14. 运输信息
14.1 UN编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 无危险货物
国际海运危规: 无危险货物
国际空运危规: 无危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别预防
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
: 4,4′-Dimethoxybenzil
化学品俗名或商品名
1.2 鉴别的其他方法
Di-p-anisoyl
p-Anisil
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
根据全球协调系统(GHS)的规定,不是危险物质或混合物。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: Di-p-anisoyl
别名
p-Anisil
: C16H14O4
分子式
: 270.28 g/mol
分子量
成分 浓度
4,4'-Dimethoxybenzil
-
化学文摘编号(CAS No.) 1226-42-2
EC-编号 214-960-5
模块 4. 急救措施
4.1 必要的急救措施描述
如果吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。
在皮肤接触的情况下
用肥皂和大量的水冲洗。
在眼睛接触的情况下
用水冲洗眼睛作为预防措施。
如果误服
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。
4.2 最重要的症状和影响,急性的和滞后的
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 救火人员的预防
如必要的话,戴自给式呼吸器去救火。
5.4 进一步的信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
防止粉尘的生成。 防止吸入蒸汽、气雾或气体。
6.2 环境预防措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
扫掉和铲掉。 存放在合适的封闭的处理容器内。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制/个体防护
8.1 控制参数
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
人身保护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所来选择人体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 结晶
颜色: 黄色
b) 气味
无数据资料
c) 气味临界值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/熔点范围: 132 - 134 °C - lit.
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 可燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 相对蒸气密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) 辛醇/水分配系数的对数值
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 化学稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 避免接触的条件
无数据资料
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤腐蚀/刺激
无数据资料
严重眼损伤 / 眼刺激
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞诱变
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 生物积累的潜在可能性
无数据资料
12.4 土壤中的迁移
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
污染了的包装物
作为未用过的产品弃置。
模块 14. 运输信息
14.1 UN编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 无危险货物
国际海运危规: 无危险货物
国际空运危规: 无危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别预防
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 脱氧茴香偶姻 1,4-di(4-methoxyphenyl)ethanone 120-44-5 C16H16O3 256.301 4-甲氧基-2-苯基苯乙酮 4-methoxyphenyl benzyl ketone 1023-17-2 C15H14O2 226.275 4,4’-二羟基苯偶酰 4,4'-dihydroxybenzil 33288-79-8 C14H10O4 242.231 2-(4-甲氧基苯基)-2-氧代乙醛 p-methoxyphenylglyoxal 1076-95-5 C9H8O3 164.161 2-氯-1,2-双-(4-甲氧基苯基)-乙酮 2-chloro-1,2-bis(4-methoxyphenyl)ethanone 71193-36-7 C16H15ClO3 290.746 茴香偶姻 4,4'-dimethoxybenzoin 119-52-8 C16H16O4 272.301 2-溴-1,2-双-(4-甲氧基苯基)-乙酮 2-bromo-1,2-bis(4-methoxyphenyl)ethanone 27895-95-0 C16H15BrO3 335.197 —— (S)-2-hydroxy-1,2-bis(4-methoxyphenyl)ethanone 119-52-8 C16H16O4 272.301 对甲氧基苯乙醛酸 p-methoxybenzoylformic acid 7099-91-4 C9H8O4 180.16 —— 4,4'-Dimethoxymonothiobenzil 71193-34-5 C16H14O3S 286.351 对甲氧基苯乙酮 1-(4-methoxyphenyl)ethanone 100-06-1 C9H10O2 150.177 —— 1,2-bis(4-methoxy-phenyl)-1,2-ethanedione 52578-09-3 C17H16O4 284.312 (4-甲氧基苯基)-氧代乙腈 4-methoxybenzoyl cyanide 14271-83-1 C9H7NO2 161.16 —— 3,4-methylenedioxybenzyl 3,4-methylenedioxyphenyl ketone 50463-29-1 C16H12O5 284.268 —— 4,4'-dimethoxylbenzil monohydrazone 40030-79-3 C16H16N2O3 284.315 —— monohydrazone of 4,4'-dimethoxybenzil 40030-79-3 C16H16N2O3 284.315 —— (p-Methoxy-benzoyl)-(p-methoxy-phenyl)-diazomethan 18627-14-0 C16H14N2O3 282.299 —— (4-Methoxyphenyl)<1-(4-methoxyphenyl)-2-dimethylaminovinyl)>keton 66521-59-3 C19H21NO3 311.381 1,3-双(4-甲氧基苯基)1,3-丙二酮 1,3-bis(4'-methoxyphenyl)propane-1,3-dione 18362-51-1 C17H16O4 284.312 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 4-hydroxy-4'-methoxybenzil 54945-19-6 C15H12O4 256.258 脱氧茴香偶姻 1,4-di(4-methoxyphenyl)ethanone 120-44-5 C16H16O3 256.301 4,4’-二羟基苯偶酰 4,4'-dihydroxybenzil 33288-79-8 C14H10O4 242.231 1,2-二(4-丁氧基苯基)乙烷-1,2-二酮 1,2-bis(4-butoxyphenyl)ethane-1,2-dione 114435-12-0 C22H26O4 354.446 —— 4,4'-bis(methoxycarboxylic acid)benzil 229623-74-9 C18H14O8 358.304 —— 4,4'-dihexyloxybenzil 1038526-42-9 C26H34O4 410.554 —— 1,2-bis(4-(dodecyloxy)phenyl)ethane-1,2-dione 159254-46-3 C38H58O4 578.876 —— 4,4'-bis[(2,2,2-trichloroethyl)methoxycarboxylate]benzil 229623-76-1 C22H16Cl6O8 621.082 茴香偶姻 4,4'-dimethoxybenzoin 119-52-8 C16H16O4 272.301 —— (R)-2-hydroxy-1,2-bis(4-methoxyphenyl)ethanone 119-52-8 C16H16O4 272.301 —— (S)-2-hydroxy-1,2-bis(4-methoxyphenyl)ethanone 119-52-8 C16H16O4 272.301 —— dibenzyl 4,4'-bis(methoxycarboxylate)benzil 229623-64-7 C32H26O8 538.554 —— 2-phenyl-2-(4'-methoxyphenyl)-4'-methoxyacetophenone 26215-78-1 C22H20O3 332.399 6-甲氧基-2-(4-苯甲氧基)苯并噻吩 1,2-bis(4-methoxyphenyl)butanone 4390-94-7 C18H20O3 284.355 4,4'-亚乙基二苯甲醚 1,2-bis(4-methoxyphenyl)ethane 1657-55-2 C16H18O2 242.318 —— 4-<2-(4-methoxyphenyl)ethyl>phenol 7329-85-3 C15H16O2 228.291 —— 4,4'-dimethoxylbenzil monohydrazone 40030-79-3 C16H16N2O3 284.315 —— monohydrazone of 4,4'-dimethoxybenzil 40030-79-3 C16H16N2O3 284.315 2,2-二(4-甲氧基苯基)-1,2-二苯基乙酮 2,2-bis-(p-methoxyphenyl)-1,2-diphenylethanone 103281-33-0 C28H24O3 408.497 —— 1-(4-methoxyphenyl)but-3-en-1-one 85234-21-5 C11H12O2 176.215 —— Methyl 3-hydroxy-5-[2-[2-[4-[2-oxo-2-(4-phenylmethoxyphenyl)acetyl]phenoxy]ethoxy]ethoxy]benzoate 885058-84-4 C33H30O9 570.596 —— 1,6-bis(4-methoxyphenyl)hexa-1,6-dione 4280-49-3 C20H22O4 326.392 —— (2Z)-1,2-bis(4-methoxyphenyl)-2-(methylhydrazono)ethanone 1023667-10-8 C17H18N2O3 298.342 —— 1,2,3-Tri-p-anisyl-2-propen-1-on 54656-03-0 C24H22O4 374.436 - 1
- 2
- 3
反应信息
-
作为反应物:描述:参考文献:名称:Sah, Journal of the Chinese Chemical Society (Peking), 1946, vol. 13, p. 111,117摘要:DOI:
-
作为产物:描述:林可霉素 2,7-二乙酸酯 在 sodium periodate 、 C54H45ClN9OsRu(3+)*3F6P(1-) 作用下, 以 水 、 丙酮 为溶剂, 反应 3.0h, 以80%的产率得到4,4'-二甲氧基苯酚酯参考文献:名称:协调助催化剂组装:助催化活性的远程O在钌方面的表现优于钌。摘要:本文介绍的是一组双金属和三金属“配位促进剂-催化剂”组件,其中配位化合物[RuII(terpy)2]和[OsII(terpy)2]充当增强[RuII(NHC)的催化活性的催化剂)(对伞花烃)为基础的催化位点。在烯烃和炔烃氧化成相应的醛,酮和二酮的过程中,助催化剂加速了对甲基苯甲基从催化部位的氧化损失,从而生成了活性催化剂。发现在这些组件中,[OsII(terpy)2]单元的增强效率明显高于其同类物[RuII(terpy)2]单元。进行了机理研究以了解这种独特的改进。DOI:10.1002/asia.201901215
文献信息
-
Fused imidazoles as potential chemical scaffolds for inhibition of heat shock protein 70 and induction of apoptosis. Synthesis and biological evaluation of phenanthro[9,10-d]imidazoles and imidazo[4,5-f][1,10]phenanthrolines
-
Vanilloid receptor ligands and their use in treatments申请人:Gore Keshav Vijay公开号:US20060084640A1公开(公告)日:2006-04-20Therapeutic benzimidazoles and compositions containing them, for the treatment of acute, inflammatory and neuropathic pain, dental pain, general headache, migraine, cluster headache, mixed-vascular and non-vascular syndromes, tension headache, general inflammation, arthritis, rheumatic diseases, osteoarthritis, inflammatory bowel disorders, inflammatory eye disorders, inflammatory or unstable bladder disorders, psoriasis, skin complaints with inflammatory components, chronic inflammatory conditions, inflammatory pain and associated hyperalgesia and allodynia, neuropathic pain and associated hyperalgesia and allodynia, diabetic neuropathy pain, causalgia, sympathetically maintained pain, deafferentation syndromes, asthma, epithelial tissue damage or dysfunction, herpes simplex, disturbances of visceral motility at respiratory, genitourinary, gastrointestinal or vascular regions, wounds, burns, allergic skin reactions, pruritus, vitiligo, general gastrointestinal disorders, gastric ulceration, duodenal ulcers, diarrhea, gastric lesions induced by necrotising agents, hair growth, vasomotor or allergic rhinitis, bronchial disorders or bladder disorders.治疗性苯并咪唑及含有它们的组合物,用于治疗急性、炎症性和神经痛、牙痛、普通头痛、偏头痛、集群头痛、混合血管和非血管综合征、紧张性头痛、一般炎症、关节炎、风湿性疾病、骨关节炎、炎症性肠道疾病、炎症性眼部疾病、炎症性或不稳定的膀胱疾病、牛皮癣、带有炎症成分的皮肤疾病、慢性炎症症状、炎症性疼痛及相关的过敏性疼痛和触痛、神经痛及相关的过敏性疼痛和触痛、糖尿病性神经病痛、烧灼性疼痛、交感神经维持性疼痛、去神经症候群、哮喘、上皮组织损伤或功能障碍、单纯疱疹、呼吸、泌尿、消化或血管区域内脏运动障碍、伤口、烧伤、过敏性皮肤反应、瘙痒、白癜风、一般胃肠道疾病、胃溃疡、十二指肠溃疡、腹泻、由坏死性剂引起的胃病变、毛发生长、血管运动性或过敏性鼻炎、支气管疾病或膀胱疾病。
-
Wet THF as a Suitable Solvent for a Mild and Convenient Reduction of Carbonyl Compounds with NaBH<sub>4</sub>作者:Behzad Zeynizadeh、Tarifeh BehyarDOI:10.1246/bcsj.78.307日期:2005.2NaBH 4 in wet THF can readily reduce varieties of carbonyl compounds such as aldehydes, ketones, conjugated enones, acyloins, and α-diketones to their corresponding alcohols in good to excellent yields. Reduction reactions were performed at room temperature or under reflux condition. In addition, the chemoselective reduction of aldehydes over ketones was accomplished successfully with this reducing湿 THF 中的 NaBH 4 可以很容易地将各种羰基化合物还原为相应的醇,例如醛、酮、共轭烯酮、酰基和 α-二酮,收率非常好。还原反应在室温或回流条件下进行。此外,使用该还原系统成功地完成了醛相对于酮的化学选择性还原。
-
Modified Hydroborate Agent: (2,2′-Bipyridyl)(tetrahydroborato)zinc Complex, [Zn(BH<sub>4</sub>)<sub>2</sub>(bpy)], as a New, Stable, Efficient Ligand-Metal Hydroborate and Chemoselective Reducing Agent作者:Behzad ZeynizadehDOI:10.1246/bcsj.76.317日期:2003.2rato)zinc complex, [Zn(BH4)2(bpy)], is a new white stable compound which has been used for efficient reduction of variety of carbonyl compounds such as aldehydes, ketones, acyloins, α-diketones and α,β-unsaturated carbonyl compounds (1,2-reduction) to their corresponding alcohols in acetonitrile at room temperature. Excellent chemoselectivity was also observed for the reduction of aldehydes over ketones
-
NaBH<sub>4</sub>/NaHSO<sub>4</sub>·H<sub>2</sub>O a Heterogeneous Acidic System for a Mild and Convenient Reduction of Carbonyl Compounds under Protic Condition作者:Behzad Zeynizadeh、Tarifeh BehyarDOI:10.1515/znb-2005-0417日期:2005.4.1
NaBH4 in the presence of sodium bisulfate (NaHSO4·H2O), a weakly acidic reagent, efficiently reduces a variety of carbonyl compounds such as aldehydes, ketones, α,β -unsaturated aldehydes and ketones, α-diketones and acyloins to their corresponding alcohols in acetonitrile under heterogeneous condition. Reduction reactions were accomplished at room temperature or under reflux condition
表征谱图
-
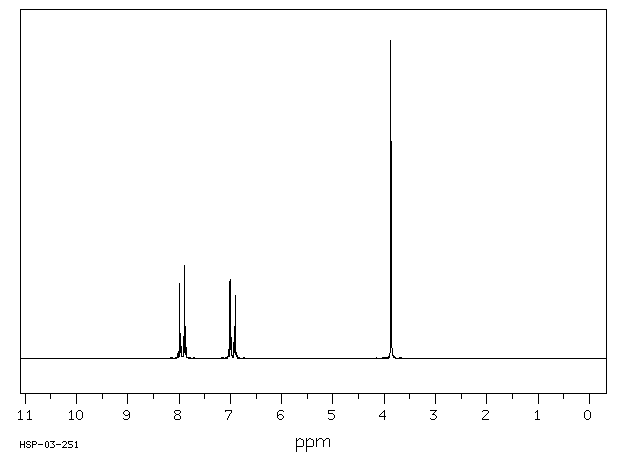
氢谱1HNMR
-
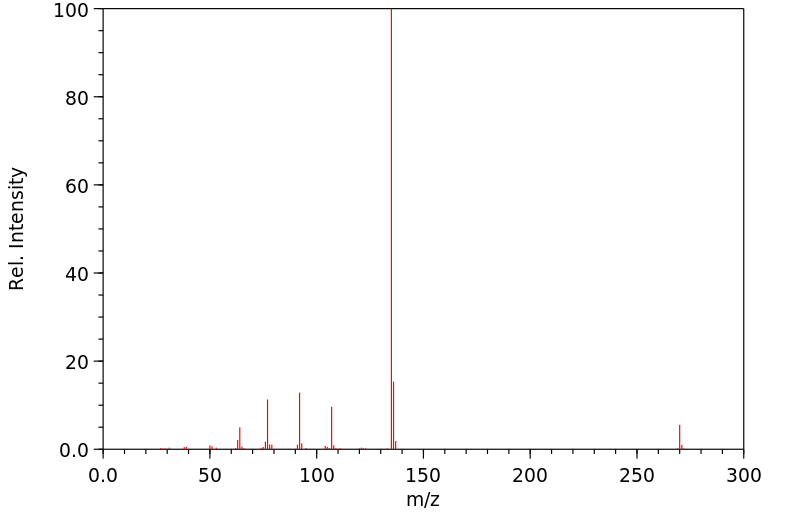
质谱MS
-
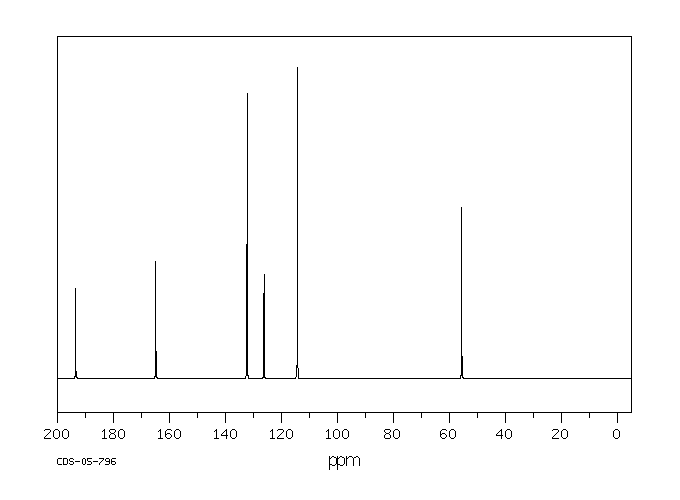
碳谱13CNMR
-
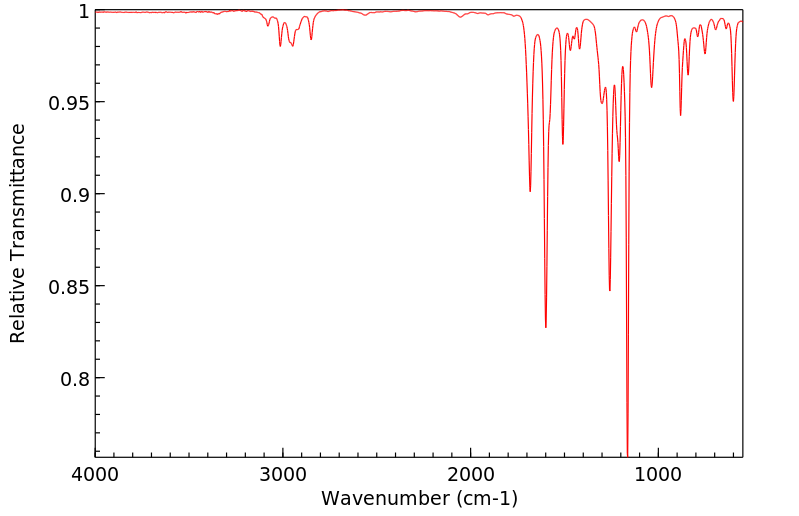
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(E,Z)-他莫昔芬N-β-D-葡糖醛酸
(E/Z)-他莫昔芬-d5
(4S,5R)-4,5-二苯基-1,2,3-恶噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S,5R,5''R)-2,2''-(1-甲基亚乙基)双[4,5-二氢-4,5-二苯基恶唑]
(4R,5S)-4,5-二苯基-1,2,3-恶噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4R,4''R,5S,5''S)-2,2''-(1-甲基亚乙基)双[4,5-二氢-4,5-二苯基恶唑]
(1R,2R)-2-(二苯基膦基)-1,2-二苯基乙胺
鼓槌石斛素
黄子囊素
高黄绿酸
顺式白藜芦醇三甲醚
顺式白藜芦醇
顺式己烯雌酚
顺式-白藜芦醇3-O-beta-D-葡糖苷酸
顺式-桑皮苷A
顺式-曲札芪苷
顺式-二苯乙烯
顺式-beta-羟基他莫昔芬
顺式-a-羟基他莫昔芬
顺式-3,4',5-三甲氧基-3'-羟基二苯乙烯
顺式-1-(3-甲基-2-萘基)-2-(2-萘基)乙烯
顺式-1,2-双(三甲基硅氧基)-1,2-双(4-溴苯基)环丙烷
顺式-1,2-二苯基环丁烷
顺-均二苯乙烯硼酸二乙醇胺酯
顺-4-硝基二苯乙烯
顺-1-异丙基-2,3-二苯基氮丙啶
非洲李(PRUNUSAFRICANA)树皮提取物
阿非昔芬
阿里可拉唑
阿那曲唑二聚体
阿托伐他汀环氧四氢呋喃
阿托伐他汀环氧乙烷杂质
阿托伐他汀环(氟苯基)钠盐杂质
阿托伐他汀环(氟苯基)烯丙基酯
阿托伐他汀杂质D
阿托伐他汀杂质94
阿托伐他汀杂质7
阿托伐他汀杂质5
阿托伐他汀内酰胺钠盐杂质
阿托伐他汀中间体M4
阿奈库碘铵
锌(II)(苯甲醛)(四苯基卟啉)
银松素
铜酸盐(5-),[m-[2-[2-[1-[4-[2-[4-[[4-[[4-[2-[4-[4-[2-[2-(羧基-kO)苯基]二氮烯基-kN1]-4,5-二氢-3-甲基-5-(羰基-kO)-1H-吡唑-1-基]-2-硫代苯基]乙烯基]-3-硫代苯基]氨基]-6-(苯基氨基)-1,3,5-三嗪-2-基]氨基]-2-硫代苯基]乙烯基]-3-硫代
铒(III) 离子载体 I
铀,二(二苯基甲酮)四碘-
钾钠2,2'-[(E)-1,2-乙烯二基]二[5-({4-苯胺基-6-[(2-羟基乙基)氨基]-1,3,5-三嗪-2-基}氨基)苯磺酸酯](1:1:1)
钠{4-[氧代(苯基)乙酰基]苯基}甲烷磺酸酯
钠;[2-甲氧基-5-[2-(3,4,5-三甲氧基苯基)乙基]苯基]硫酸盐
钠4-氨基二苯乙烯-2-磺酸酯