4,5-二溴-1,2-二甲氧基苯 | 37895-73-1
物质功能分类
中文名称
4,5-二溴-1,2-二甲氧基苯
中文别名
4,5-二溴藜芦醚
英文名称
1,2-dibromo-4,5-dimethoxybenzene
英文别名
4,5-dibromoveratrole;4,5-dibromo-1,2-dimethoxybenzene;4,5-dibromoveratrol;1,2-dimethoxy-4,5-dibromobenzene
CAS
37895-73-1
化学式
C8H8Br2O2
mdl
MFCD00014894
分子量
295.958
InChiKey
ZYCLQXMMFJREPJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:90-92°C
-
沸点:286.6±35.0 °C(Predicted)
-
密度:1.742±0.06 g/cm3(Predicted)
-
稳定性/保质期:
常规情况下不会分解,也没有危险反应。
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:12
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:18.5
-
氢给体数:0
-
氢受体数:2
安全信息
-
安全说明:S22,S24/25
-
海关编码:2909309090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:密封、阴凉、干燥保存
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product identifiers
Product name : 1,2-DIBROMO-4,5-DIMETHOXYBENZENE
CAS-No. : 37895-73-1
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008.
This substance is not classified as dangerous according to Directive 67/548/EEC.
Label elements
This substance is not classified as dangerous according to Directive 67/548/EEC.
Other hazards - none
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Formula : C8H8Br2O2
Molecular Weight : 295,96 g/mol
Section 4. FIRST AID MEASURES
Description of first aid measures
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration.
In case of skin contact
Wash off with soap and plenty of water.
In case of eye contact
Flush eyes with water as a precaution.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water.
Most important symptoms and effects, both acute and delayed
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Indication of any immediate medical attention and special treatment needed
no data available
Section 5. FIREFIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides, Hydrogen bromide gas
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Avoid dust formation. Avoid breathing vapors, mist or gas.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Sweep up and shovel. Keep in suitable, closed containers for disposal.
Reference to other sections
For disposal see section 13.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Provide appropriate exhaust ventilation at places where dust is formed.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Specific end uses
no data available
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
General industrial hygiene practice.
Personal protective equipment
Eye/face protection
Use equipment for eye protection tested and approved under appropriate government standards
such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
Choose body protection in relation to its type, to the concentration and amount of dangerous
substances, and to the specific work-place., The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Respiratory protection is not required. Where protection from nuisance levels of dusts are desired,
use type N95 (US) or type P1 (EN 143) dust masks. Use respirators and components tested and
approved under appropriate government standards such as NIOSH (US) or CEN (EU).
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
a) Appearance Form: solid
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing no data available
point
f) Initial boiling point and no data available
boiling range
g) Flash point no data available
h) Evaporation rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility no data available
o) Partition coefficient: n- log Pow: 3,395
octanol/water
p) Autoignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
Section 10. STABILITY AND REACTIVITY
Reactivity
no data available
Chemical stability
no data available
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Section 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Potential health effects
Inhalation May be harmful if inhaled. May cause respiratory tract irritation.
Ingestion May be harmful if swallowed.
Skin May be harmful if absorbed through skin. May cause skin irritation.
Eyes May cause eye irritation.
Signs and Symptoms of Exposure
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Additional Information
RTECS: Not available
Section 12. ECOLOGICAL INFORMATION
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
no data available
Other adverse effects
no data available
Section 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
SECTION 15 - REGULATORY INFORMATION
N/A
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 4,5-dibromo-2-methoxyphenol 38926-86-2 C7H6Br2O2 281.931 4-溴黎芦醚 4-Bromoveratrole 2859-78-1 C8H9BrO2 217.062 4,5-二溴苯-1,2-二醇 4,5-dibromobenzene-1,2-diol 2563-26-0 C6H4Br2O2 267.905 2-溴-4,5-二乙氧基苯胺 2-bromo-4,5-dimethoxyaniline 16791-41-6 C8H10BrNO2 232.077 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1,2-溴-4,5-亚甲二氧基苯 5,6-dibromo-1,3-benzodioxole 5279-32-3 C7H4Br2O2 279.916 苯,1,2-二溴-4,5-二(己氧基)- 1,2-dibromo-4,5-bis(hexyloxy)benzene 118132-03-9 C18H28Br2O2 436.227 1,2,3-三溴-4,5-二甲氧基苯 2,3,4-tribromoveratrole 854872-30-3 C8H7Br3O2 374.854 4,5-二溴苯-1,2-二醇 4,5-dibromobenzene-1,2-diol 2563-26-0 C6H4Br2O2 267.905 1,2,3,4-四溴-5,6-二甲氧基苯 tetrabromoveratrole 26884-57-1 C8H6Br4O2 453.75 —— 4,5-dibromo-3,6-diiodoveratrole 120231-43-8 C8H6Br2I2O2 547.751 —— 2,3-dibromo-9,10-dimethoxypentacene 1374265-71-0 C24H16Br2O2 496.198
反应信息
-
作为反应物:描述:4,5-二溴-1,2-二甲氧基苯 在 tetrabutylammonium tetrafluoroborate 、 三乙胺 作用下, 以 四氢呋喃 、 乙腈 为溶剂, 反应 4.4h, 以77%的产率得到邻苯二甲醚参考文献:名称:通过直接电解对芳基卤化物进行加氢脱卤摘要:公开了无催化剂和无金属的芳基卤化物的电化学加氢脱卤。我们通过灵活的协议进行的反应在配备了廉价石墨棒阳极和阴极的不分隔电池中进行。三烷基胺n Bu 3 N / Et 3 N充当该电化学还原反应的有效还原剂和氢原子供体。各种芳基和杂芳基溴有效地起作用。通常反应性较低的芳基氯化物和氟化物也可以顺利转化。克农药规模的有害农药排毒和二溴代联苯的加氢溴化(阻燃剂类似物)证明了我们方法的实用性。DOI:10.1002/chem.201901082
-
作为产物:参考文献:名称:Mono-oxo-bis-dithioveratrol-molybdate - 在溶液中是亚砷酸氧化酶的模型,在固态中是具有前所未有的结合基序的配位聚合物摘要:Mono-oxo-bis-dithioveratrol-molybdate 被合成、结构表征和研究其氧化转移活性。后者与类似的钨配合物的活性进行了比较。标题复合物是含有亚砷酸氧化酶的钼蝶呤的结构模型,两种复合物均成功催化氧转移反应达到 100% 转化率。使用二硫藜芦醇配体,对于钨络合物,三苯基膦的氧化被证明更快,这是不典型的。钼复合物的固态结构表现出意想不到且非常不寻常的聚合物结构基序,由复合阴离子单氧代双二硫藜芦醇钼酸盐、钠阳离子和甲醇组成。DOI:10.1002/zaac.201300076
文献信息
-
Synthesis of Fluorene and Indenofluorene Compounds: Tandem Palladium-Catalyzed Suzuki Cross-Coupling and Cyclization作者:Tao-Ping Liu、Chun-Hui Xing、Qiao-Sheng HuDOI:10.1002/anie.201000327日期:2010.4.6“Fluor” it: Palladium‐catalyzed tandem reactions, in which C(sp3)H bond activation is the key step (see scheme; DMA=dimethylacetamide), lead to substituted fluorenes and indenofluorenes through annulation in high yield and in one step. This method has potential for the preparation of other cyclic compounds, as well as substituted oligofluorenes and polyfluorenes.
-
Iridium-Catalyzed Asymmetric Ring-Opening of Oxabenzonorbornadienes with N-Substituted Piperazine Nucleophiles作者:Wen Yang、Renshi Luo、Dingqiao YangDOI:10.3390/molecules201219748日期:——Iridium-catalyzed asymmetric ring-opening of oxabenzonorbornadienes with N-substituted piperazines was described. The reaction afforded the corresponding ring-opening products in high yields and moderate enantioselectivities in the presence of 2.5 mol % [Ir(COD)Cl]2 and 5.0 mol % (S)-p-Tol-BINAP. The effects of various chiral bidentate ligands, catalyst loading, solvent, and temperature on the yield and enantioselectivity were also investigated. A plausible mechanism was proposed to account for the formation of the corresponding trans-ring opened products based on the X-ray structure of product 2i.
-
Dehydroxymethyl Bromination of Alkoxybenzyl Alcohols by Using a Hypervalent Iodine Reagent and Lithium Bromide作者:Tomohiro Maegawa、Ayako Shibata、Sara Kitamoto、Kazuma Fujimura、Yuuka Hirose、Hiromi Hamamoto、Akira Nakamura、Yasuyoshi MikiDOI:10.1055/s-0037-1610980日期:2018.10We describe the dehydroxymethylbromination of alkoxybenzyl alcohol by using a hypervalent iodine reagent and lithium bromide in F3CCH2OH at room temperature. Selective monobromination or dibromination was possible by adjusting the molar ratios of hypervalent iodine reagent and lithium bromide.
-
Directing Selectivity to Aldehydes, Alcohols, or Esters with Diphobane Ligands in Pd-Catalyzed Alkene Carbonylations作者:Dillon W. P. Tay、James D. Nobbs、Srinivasulu Aitipamula、George J. P. Britovsek、Martin van MeursDOI:10.1021/acs.organomet.1c00228日期:2021.6.28trifluoromethylphenylene-bridged diphobane L1 with an electron-withdrawing substituent, lead to ester products via alkoxycarbonylation, whereas BCOPE gives predominantly alcohol products (n-nonanol and isomers) via reductive hydroformylation. The preference of BCOPE for reductive hydroformylation is also seen in the hydroformylation of 1-hexene in diglyme as the solvent, producing heptanol as the major product, whereas已经合成了具有不同取代基(CF 3、H、OMe、(OMe)2、t Bu)的亚苯基桥连二恶烷配体,并将其用作钯催化的各种烯烃羰基化反应中的配体。已经使用 1-己烯、1-辛烯和戊烯酸甲酯作为底物研究了这些配体在加氢甲酰化与烷氧基羰基化选择性方面的性能,并将结果与乙烯桥连二恶烷配体 ( BCOPE ) 进行了比较。1-辛烯在质子溶剂 2-乙基己醇中的加氢甲酰化导致加氢甲酰化和烷氧基羰基化之间的竞争,其中亚苯基桥连的配体,特别是三氟甲基亚苯基桥连的二恶烷L1带有吸电子取代基,通过烷氧基羰基化产生酯产物,而BCOPE通过还原加氢甲酰化产生主要的醇产物(正壬醇和异构体)。在以二甘醇二甲醚为溶剂的 1-己烯的加氢甲酰化中也可以看到BCOPE对还原加氢甲酰化的偏好,生成庚醇作为主要产物,而在这种情况下,亚苯基桥连的配体显示出低得多的活性。亚苯基桥连配体在 1-辛烯甲氧基羰基化生成壬酸甲酯方面表现出优异
-
Diastereoselective [3+2] Annulation of Aromatic/Vinylic Amides with Bicyclic Alkenes through Cobalt-Catalyzed C−H Activation and Intramolecular Nucleophilic Addition作者:Parthasarathy Gandeepan、Pachaiyappan Rajamalli、Chien-Hong ChengDOI:10.1002/anie.201512018日期:2016.3.18dihydroepoxybenzofluorenone derivatives from aromatic/vinylic amides and bicyclic alkenes is described. This new transformation proceeds through cobalt‐catalyzed C−H activation and intramolecular nucleophilic addition to the amide functional group. Transition‐metal‐catalyzed C−H activation reactions of secondary amides with alkenes usually lead to [4+2] or [4+1] annulation; to the best of our knowledge
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
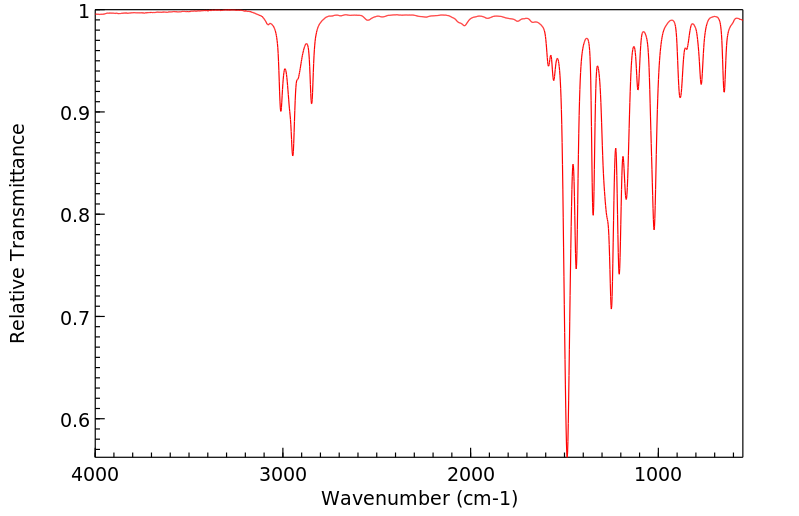
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫