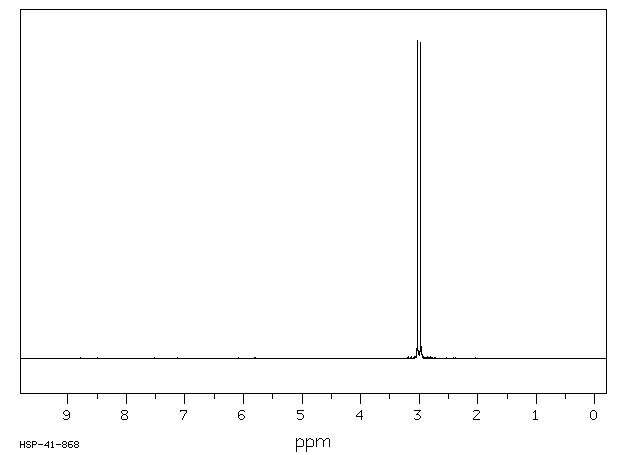
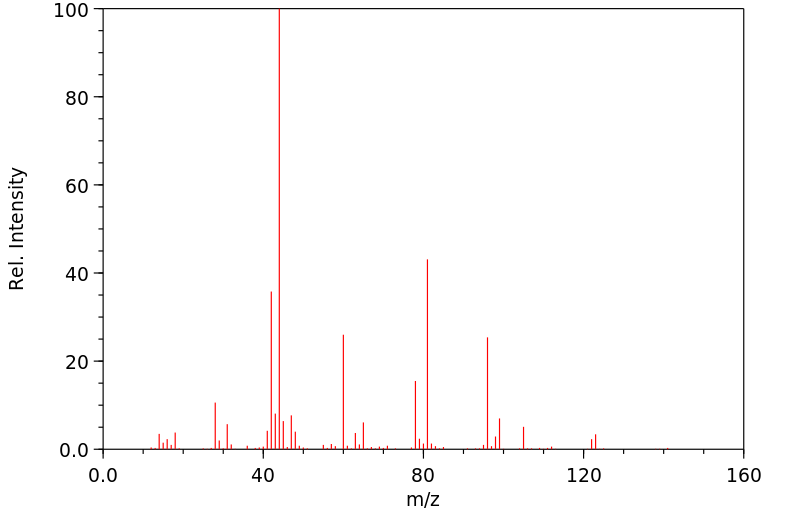
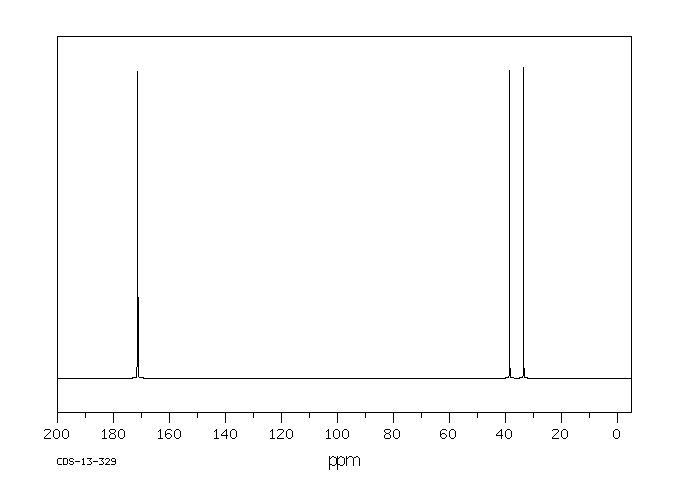
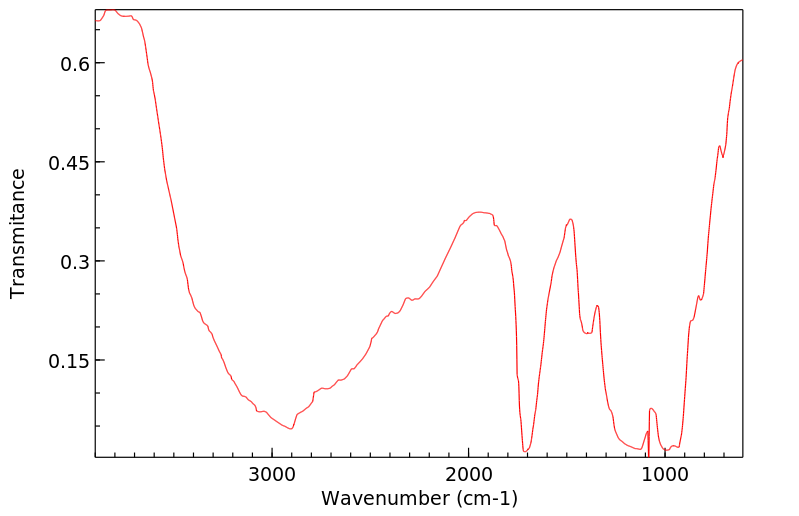
毒理性
Dermatotoxin - 皮肤烧伤。
Dermatotoxin - Skin burns.
来源:Haz-Map, Information on Hazardous Chemicals and Occupational Diseases