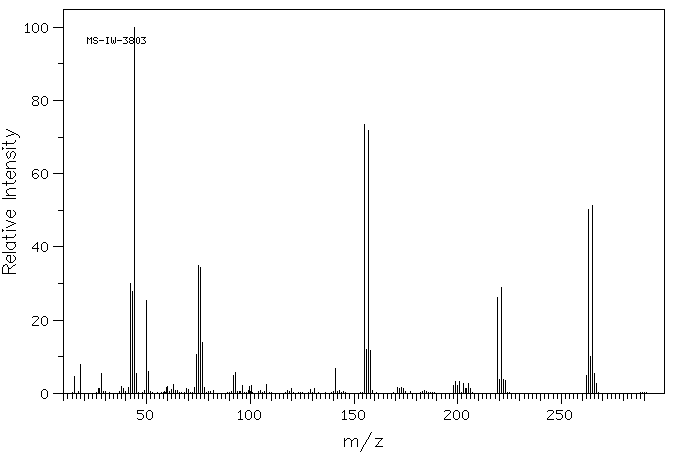
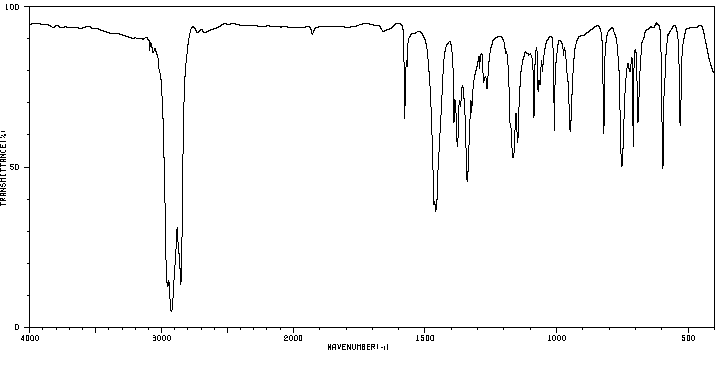
4-溴-N,N-二甲基苯磺酰胺 | 707-60-8
中文名称
4-溴-N,N-二甲基苯磺酰胺
中文别名
N,N-二甲基-4-溴苯磺酰胺;4-溴-N,N-二甲基苯磺胺;N,N-二甲基对溴苯磺酰胺
英文名称
4-bromo-N,N-dimethylbenzenesulfonamide
英文别名
4-bromo-N,N-dimethylbenzene-1-sulfonamide;N,N-dimethyl-4-bromophenylsulfonamide;N,N-dimethyl-4-bromobenzenesulfonamide
CAS
707-60-8
化学式
C8H10BrNO2S
mdl
MFCD00017834
分子量
264.143
InChiKey
NQAUNPZZVCXYEJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:88-90
-
沸点:329.0±44.0 °C(Predicted)
-
密度:1.543±0.06 g/cm3(Predicted)
-
稳定性/保质期:
远离氧化物。
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:13
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:45.8
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
海关编码:2935009090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:存放在密封容器中,并置于阴凉、干燥处。请确保储存地点远离氧化剂。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
N,N-Dimethyl 4-bromobenzenesulfonamide
Product Name:
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
Wear protective gloves/protective clothing/eye protection/face protection
P280:
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
P304+P340: IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing
P405: Store locked up
Section 3. Composition/information on ingredients.
N,N-Dimethyl 4-bromobenzenesulfonamide
Ingredient name:
CAS number: 707-60-8
Section 4. First aid measures
Immediately wash skin with copious amounts of water for at least 15 minutes while removing
Skin contact:
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Ingestion:
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Not specified
Appearance:
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C8H10BrNO2S
Molecular weight: 264.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen bromide, sulfur oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
N,N-Dimethyl 4-bromobenzenesulfonamide
Product Name:
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
Wear protective gloves/protective clothing/eye protection/face protection
P280:
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
P304+P340: IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing
P405: Store locked up
Section 3. Composition/information on ingredients.
N,N-Dimethyl 4-bromobenzenesulfonamide
Ingredient name:
CAS number: 707-60-8
Section 4. First aid measures
Immediately wash skin with copious amounts of water for at least 15 minutes while removing
Skin contact:
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Ingestion:
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Not specified
Appearance:
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C8H10BrNO2S
Molecular weight: 264.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen bromide, sulfur oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-溴苯磺酰胺 4-bromo-benzenesulfonic acid amide 701-34-8 C6H6BrNO2S 236.089 4-氨基-N,N-二甲基苯磺酰胺 4-amino-N,N-dimethylbenzenesulphonamide 1709-59-7 C8H12N2O2S 200.261 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N,N-二甲基-苯磺酰胺 N,N-dimethylbenzenesulfonamide 14417-01-7 C8H11NO2S 185.247 —— 4-mercapto-N,N-dimethylbenzenesulfonamide 18325-52-5 C8H11NO2S2 217.313 —— N,N-dimethyl-4-vinylbenzenesulfonamide 60472-40-4 C10H13NO2S 211.285 —— 4-(N,N-dimethylsulfamoyl)benzenesulfonyl chloride 677782-39-7 C8H10ClNO4S2 283.757 N,N-二甲基-4-硼苯磺酰胺 {4-[(dimethylamino)sulfonyl]phenyl}boronic acid 486422-59-7 C8H12BNO4S 229.065
反应信息
-
作为反应物:描述:4-溴-N,N-二甲基苯磺酰胺 在 sodium periodate 、 1,1'-双(二苯膦基)二茂铁二氯化钯(II)二氯甲烷复合物 、 potassium acetate 作用下, 以 四氢呋喃 、 1,4-二氧六环 、 水 为溶剂, 反应 17.58h, 生成 N,N-二甲基-4-硼苯磺酰胺参考文献:名称:WO2020024017A5摘要:公开号:WO2020024017A5
-
作为产物:描述:4-氨基-N,N-二甲基苯磺酰胺 在 三氯溴甲烷 、 sodium nitrite 、 溶剂黄146 作用下, 以 二氯甲烷 、 水 为溶剂, 反应 1.08h, 以98%的产率得到4-溴-N,N-二甲基苯磺酰胺参考文献:名称:一锅,无金属地将苯胺转化为芳基溴化物和碘化物摘要:描述了通过从溴代三氯甲烷和二碘代甲烷中提取卤素从苯胺无金属合成芳基溴化物和碘化物。该一锅法反应以中等至优异的收率从相应的苯胺提供了卤代芳基卤化物,而没有分离出重氮盐。该转化反应时间短,后处理简单,对湿气和空气不敏感,并且避免了过多的卤化。DFT计算支持S RN 1机制。此方法代表了经典Sandmeyer反应的一种方便替代方法。DOI:10.1021/acs.orglett.7b00771
文献信息
-
DIHYDROPYRIDAZINE-3,5-DIONE DERIVATIVE AND PHARMACEUTICALS CONTAINING THE SAME申请人:CHUGAI SEIYAKU KABUSHIKI KAISHA公开号:US20160002251A1公开(公告)日:2016-01-07The present invention provides a dihydropyridazine-3,5-dione derivative or a salt thereof, or a solvate of the compound or the salt, a pharmaceutical drug, a pharmaceutical composition, a sodium-dependent phosphate transporter inhibitor, and a preventive and/or therapeutic agent for hyperphosphatemia, secondary hyperparathyroidism, chronic renal failure, chronic kidney disease, and arteriosclerosis associated with vascular calcification comprising the compound as an active ingredient, and a method for prevention and/or treatment.
-
[EN] SELECTIVE ESTROGEN RECEPTOR MODULATORS CONTAINING A PHENYLSULFONYL GROUP<br/>[FR] MODULATEURS SELECTIFS DES RECEPTEURS OESTROGENIQUES CONTENANT UN GROUPE PHENYLSULFONYLE申请人:LILLY CO ELI公开号:WO2004009086A1公开(公告)日:2004-01-29The present invention relates to a selective estrogen receptor modulator of formula I or a pharmaceutical acid addition salt thereof; useful, e.g., for treating endometriosis and/or uterine leiomyoma/leiomyomata.本发明涉及式I的选择性雌激素受体调节剂或其药用酸盐加合物;例如,用于治疗子宫内膜异位症和/或子宫平滑肌瘤。
-
Potassium tert-butoxide-mediated metal-free synthesis of sulfonamides from sodium sulfinates and N,N-disubstituted formamides作者:Xiaodong Bao、Xiaona Rong、Zhiguo Liu、Yugui Gu、Guang Liang、Qinqin XiaDOI:10.1016/j.tetlet.2018.06.031日期:2018.7By using formamides as amine sources, a novel and efficient KO-t-Bu mediated amination of sodium sulfinates has been developed. The reaction utilizes readily available starting materials under metal-free conditions, thus providing an alternative and attractive route to sulfonamides.
-
Biphenylsulfonamide Endothelin Antagonists: Structure−Activity Relationships of a Series of Mono- and Disubstituted Analogues and Pharmacology of the Orally Active Endothelin Antagonist 2‘-Amino-<i>N</i>- (3,4-dimethyl-5-isoxazolyl)-4‘-(2-methylpropyl)[1,1‘-biphenyl]-2-sulfonamide (BMS-187308)作者:Natesan Murugesan、Zhengxiang Gu、Philip D. Stein、Sharon Bisaha、Steve Spergel、Ravi Girotra、Ving G. Lee、John Lloyd、Raj N. Misra、Joan Schmidt、Arvind Mathur、Leslie Stratton、Yolanda F. Kelly、Eileen Bird、Tom Waldron、Eddie C.-K. Liu、Rongan Zhang、Helen Lee、Randy Serafino、Benoni Abboa-Offei、Parker Mathers、Mary Giancarli、Andrea Ann Seymour、Maria L. Webb、Suzanne Moreland、Joel C. Barrish、John T. HuntDOI:10.1021/jm970872k日期:1998.12.1Substitution at the ortho position of N-(3,4-dimethyl-5-isoxazolyl) benzenesulfonamide led to the identification of the biphenylsulfonamides as a novel series of endothelin-A (ETA) selective antagonists. Appropriate substitutions on the pendant phenyl ring led to improved binding as well as functional activity. A hydrophobic group such as isobutyl or isopropoxyl was found to be optimal at the 4'-position
-
[EN] NAPHTHYLACETIC ACIDS<br/>[FR] ACIDES NAPHTYLACÉTIQUES申请人:HOFFMANN LA ROCHE公开号:WO2010055005A1公开(公告)日:2010-05-20The invention is concerned with the compounds of formula (I) and pharmaceutically acceptable salts and esters thereof, wherein X, Q, and R1-R6 are defined in the detailed description and claims. In addition, the present invention relates to methods of manufacturing and using the compounds of formula (I) as well as pharmaceutical compositions containing such compounds. The compounds of formula (I) are antagonists or partial agonists at the CRTH2 receptor and may be useful in treating diseases and disorders associated with that receptor such as asthma.本发明涉及式(I)的化合物以及药用可接受的盐和酯,其中X、Q和R1-R6在详细描述和权利要求中定义。此外,本发明还涉及制造和使用式(I)化合物的方法,以及包含此类化合物的药物组合物。式(I)的化合物是CRTH2受体的拮抗剂或部分激动剂,可用于治疗与该受体相关的疾病和失调,如哮喘。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫