4-羟基-3-甲基-2-丁酮 | 3393-64-4
中文名称
4-羟基-3-甲基-2-丁酮
中文别名
——
英文名称
4-hydroxy-3-methyl-2-butanone
英文别名
4-hydroxy-3-methylbutan-2-one
CAS
3393-64-4
化学式
C5H10O2
mdl
MFCD00004739
分子量
102.133
InChiKey
VVSRECWZBBJOTG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:2.5°C (estimate)
-
沸点:90-95 °C/15 mmHg(lit.)
-
密度:0.993 g/mL at 25 °C(lit.)
-
闪点:79 °C
-
LogP:-0.440 (est)
-
保留指数:826;832
-
稳定性/保质期:
在常温常压下保持稳定。
计算性质
-
辛醇/水分配系数(LogP):-0.2
-
重原子数:7
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.8
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
海关编码:2914400090
-
安全说明:S24/25
-
储存条件:常温、避光、通风干燥处,密封保存。
SDS
| Name: | 4-Hydroxy-3-methyl-2-butanone tech. ca. 85% Material Safety Data Sheet |
| Synonym: | 4-Hydroxy-3-methylbutan-2-on |
| CAS: | 3393-64-4 |
Synonym:4-Hydroxy-3-methylbutan-2-on
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 3393-64-4 | 4-Hydroxy-3-methyl-2-butanone, tech. | 85 | 222-238-6 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation. May cause chemical conjunctivitis and corneal damage.
Skin:
May cause irritation and dermatitis. May cause cyanosis of the extremities.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. Aspiration may lead to pulmonary edema. Inhalation at high concentrations may cause CNS depression and asphixiation.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Use water spray to keep fire-exposed containers cool. Combustible liquid.
Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. Containers may explode when heated.
Extinguishing Media:
Use water spray to cool fire-exposed containers. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Remove all sources of ignition. Absorb spill using an absorbent, non-combustible material such as earth, sand, or vermiculite. Do not use combustible materials such as sawdust. Use a spark-proof tool. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use only in a well-ventilated area. Avoid contact with eyes, skin, and clothing. Empty containers retain product residue, (liquid and/or vapor), and can be dangerous. Keep container tightly closed. Keep away from heat, sparks and flame. Avoid ingestion and inhalation. Do not pressurize, cut, weld, braze, solder, drill, grind, or expose empty containers to heat, sparks or open flames.
Storage:
Keep away from heat, sparks, and flame. Keep away from sources of ignition. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate general or local explosion-proof ventilation to keep airborne levels to acceptable levels.
Exposure Limits CAS# 3393-64-4: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Clear liquid
Color: clear, colorless
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 90.0 - 95.0 deg C @ 15.00mmHg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not applicable.
Flash Point: 78 deg C ( 172.40 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: Not available.
Solubility in water: Not available.
Specific Gravity/Density: .9930g/cm3
Molecular Formula: C5H10O2
Molecular Weight: 102.13
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, ignition sources, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Strong oxidizing agents, strong bases, reducing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 3393-64-4 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
4-Hydroxy-3-methyl-2-butanone, tech. - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Other No information available.
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 3393-64-4: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 3393-64-4 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 3393-64-4 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-羟基-3-(羟基甲基)-3-甲基-2-丁酮 3-oxo-2,2-bis-(hydroxymethyl)butane 6868-97-9 C6H12O3 132.159 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3-甲基-2-丁酮 3-methyl-butan-2-one 563-80-4 C5H10O 86.1338 —— 3,4-dihydroxy-3-methyl-2-butanone —— C5H10O3 118.133
反应信息
-
作为反应物:描述:参考文献:名称:全反式无环类异戊二烯信息素成分的合成摘要:全反式无环类异戊二烯骨架是通过两步迭代序列制成的。该方法涉及由烯丙基醇形成的烯丙基乙烯基醚的克莱森重排和甲基异丙烯基酮的二甲基乙缩醛,然后通过重排形成的LiAlH 4还原α,β-不饱和酮。通过使用一锅脱氧反应来合成(E) -β-法呢烯,(E) -β-弹簧烯和树突蛋白,α,β-不饱和酮也被转化为2-甲基-1-丙烯基。DOI:10.1016/s0040-4020(01)86580-7
-
作为产物:描述:参考文献:名称:508. 2-甲基丁-2-烯亚硝基氯化物及其衍生物摘要:DOI:10.1039/jr9560002587
文献信息
-
Erbium(III) Triflate as an Extremely Active Acylation Catalyst作者:Antonio Procopio、Renato Dalpozzo、Antonio De?Nino、Loredana Maiuolo、Beatrice Russo、Giovanni SindonaDOI:10.1002/adsc.200404132日期:2004.10Erbium(III) triflate is a powerful catalyst for the acylation of alcohols and phenols. The reaction works well for a large variety of simple and functionalized substrates by using different kinds of acidic anhydrides Ac2O, (EtCO)2O, [(CH3)3CO]2O, Bz2O, and (CF3CO)2O} without isomerisation of chiral centres. Moreover, the catalyst can be easily recycled and reused without significant loss of activity
-
[EN] DITHIOLAN-3-YLPENTANOATE DERIVATIVES, PHARMACEUTICAL COMPOSITIONS AND METHODS FOR THE TREATMENT OF PAIN<br/>[FR] DÉRIVÉS DE DITHIOLAN-3-YLPENTANOATE, COMPOSITIONS PHARMACEUTIQUES ET MÉTHODES DE TRAITEMENT DE LA DOULEUR申请人:KRISANI BIOSCIENCES P LTD公开号:WO2015118554A1公开(公告)日:2015-08-13The disclosure herein provides 1-carbamoyloxyethyl 5-(1,2-dithiolan-3-yl)pentanoate derivatives of formula I, formula II and formula III. The disclosure also provides a method of synthesizing the compound of formula I, formula II and formula III. The compound of formula I, formula II and formula III or its pharmaceutical acceptable salts, as well as polymorphs, solvates, and hydrates, thereof may be formulated as pharmaceutical composition. The pharmaceutical composition of compound of formula I, formula II and formula III or the final compound of formula I, formula II or formula III may be formulated for non-invasive peroral, topical (example transdermal), enteral, transmucosal, targeted delivery, sustained release delivery, delayed release, pulsed release and parenteral methods. Such compositions may be used to treat chronic pain manifested with chronic diseases or its associated complications.
-
Erbium(III) Chloride: a Very Active Acylation Catalyst作者:Renato Dalpozzo、Antonio De Nino、Loredana Maiuolo、Manuela Oliverio、Antonio Procopio、Beatrice Russo、Amedeo TocciDOI:10.1071/ch06346日期:——Erbium(iii) chloride is a powerful catalyst for the acylation of alcohols and phenols. The reaction works well for a large variety of simple and functionalized substrates by using different kinds of acidic anhydrides (Ac2O, (EtCO)2O, (PriCO)2O, (ButCO)2O, and (CF3CO)2), without isomerization of chiral centres. Moreover, the catalyst can be easily recycled and reused without significant loss of activity
-
Metal-Catalyzed Rearrangement of Homoallylic Ethers to Silylmethyl Allylic Silanes in the Presence of a Di-<i>tert</i>-butylsilylene Source作者:Pamela A. Cleary、K. A. WoerpelDOI:10.1021/ol052456x日期:2005.11.1transfer to gem-disubstituted alkenes to form silacyclopropanes, we discovered an unprecedented reaction of homoallylic ethers. When silylene transfer was performed at room temperature or above, two di-tert-butylsilylene units were incorporated into the molecule, and complete rearrangement of the carbon backbone occurred. This report describes the scope of this unique reaction as well as the mechanistic
-
POLYMERIZABLE COMPOUND, POLYMERIZABLE COMPOSITION, POLYMER, AND PHOTORESIST COMPOSITION申请人:JNC CORPORATION公开号:US20210009546A1公开(公告)日:2021-01-14A polymerizable compound represented by formula (1). In formula (1), one of eight hydrogen atoms is substituted by a (meth)acryloyloxy group, and the rest seven hydrogen atoms are independently non-substituted or substituted by a saturated hydrocarbon group having 1 to 10 carbons.公式(1)所代表的可聚合化合物。在公式(1)中,八个氢原子中的一个被(甲基)丙烯酰氧基取代,其余七个氢原子独立地未被取代或被具有1至10个碳原子的饱和碳氢基团取代。
表征谱图
-
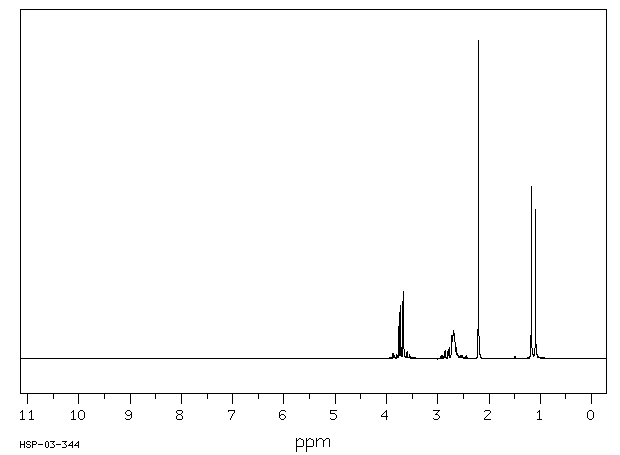
氢谱1HNMR
-
质谱MS
-
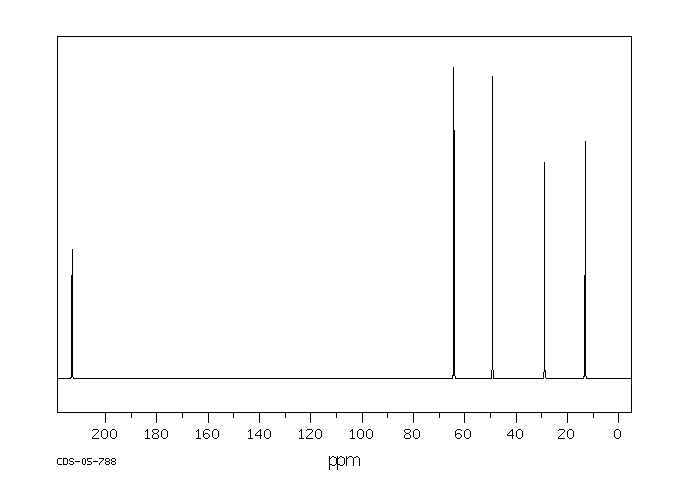
碳谱13CNMR
-
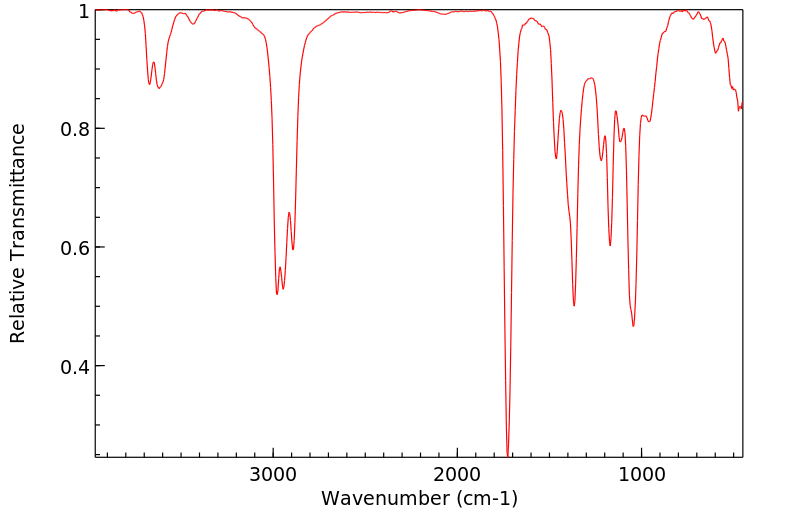
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷