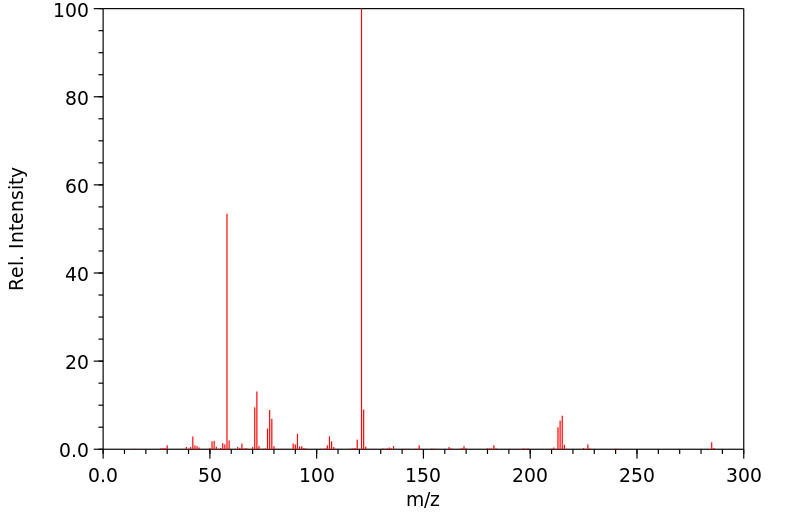
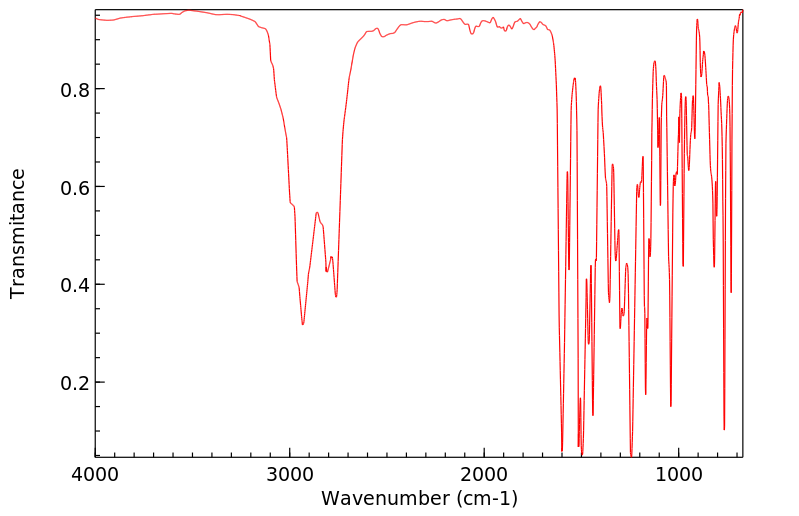
代谢
来源:Hazardous Substances Data Bank (HSDB)
代谢
H1受体拮抗剂是诱导肝微粒体酶的许多药物之一,它们可能促进自身的代谢。/
组胺拮抗剂:H1拮抗剂/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
哺乳期使用总结:在哺乳期间,偶尔使用小剂量的
吡咯胺可能是可接受的。较大剂量或更长时间的使用可能会对婴儿产生影响或减少乳汁供应,尤其是与伪
麻黄碱等拟交感神经药联合使用或在哺乳尚未建立时。首选非镇静
抗组胺药作为替代。
对哺乳婴儿的影响:截至修订日期,未找到关于
吡咯胺的相关已发布信息。在一项电话随访研究中,母亲报告有10%的婴儿接触到各种
抗组胺药后出现烦躁和腹痛症状,1.6%的婴儿出现嗜睡。所有反应均无需医疗注意,且没有婴儿接触过
吡咯胺。
对哺乳和乳汁的影响:在非哺乳期妇女和产后早期妇女中,相对高剂量的
抗组胺药通过注射可以降低基础血清
催乳素。然而,哺乳诱导的
催乳素分泌不受产后母亲
抗组胺药预处理的影响。尚未研究较低口服剂量的
抗组胺药是否对血清
催乳素有相同的影响,或者
催乳素的影响是否对哺乳成功有任何后果。在已建立哺乳的母亲中,
催乳素水平可能不会影响她的哺乳能力。
来源:Drugs and Lactation Database (LactMed)
毒理性
同时使用具有耳毒性的药物和
抗组胺药可能会掩盖耳毒性的症状,如耳鸣、头晕或眩晕。/
抗组胺药/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
单胺氧化酶(MAO)
抑制剂与
抗组胺药的并用可能会延长并增强
抗组胺药的抗
胆碱能和中枢神经系统抑制作用;不建议同时使用。/
抗组胺药/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
与
酒精或其他产生中枢神经系统抑制的药物同时使用可能会增强这些药物或
抗组胺药的中枢神经系统抑制作用;此外,与
马普替林或
三环类抗抑郁药同时使用可能会增强
抗组胺药或这些药物的的抗
胆碱能效果。/
抗组胺药/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
抗
胆碱能效果可能会在使用
抗组胺药时与具有抗
胆碱能活性的抗
胆碱药或其他药物同时使用时增强;患者应被告知及时报告胃肠道问题的发生,因为在使用联合疗法时可能会出现麻痹性肠梗阻。/
抗组胺药/
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
H1拮抗剂从胃肠道吸收良好。口服给药后,血浆浓度在2到3小时内达到峰值,效果通常持续4到6小时;然而,一些药物的药效持续更长时间... /
组胺拮抗剂:H1拮抗剂/
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
H1拮抗剂在儿童体内的消除速度比成人快,而在严重肝病患者体内的消除速度则较慢。/
组胺拮抗剂:H1拮抗剂/
来源:Hazardous Substances Data Bank (HSDB)