physostigmine | 57-47-6
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:102-104 °C(lit.)
-
比旋光度:D17 -76° (c = 1.3 in chloroform); D25 -120° (benzene)
-
沸点:418.29°C (rough estimate)
-
密度:1.166±0.06 g/cm3 (20 ºC 760 Torr)
-
闪点:>100℃
-
溶解度:氯仿(微溶、超声处理)、DMSO(微溶)、乙醇(微溶)、甲醇
计算性质
-
辛醇/水分配系数(LogP):0.7
-
重原子数:20
-
可旋转键数:2
-
环数:3.0
-
sp3杂化的碳原子比例:0.53
-
拓扑面积:44.8
-
氢给体数:1
-
氢受体数:4
安全信息
-
TSCA:Yes
-
危险等级:6.1(a)
-
危险品标志:T+
-
安全说明:S23,S25,S45
-
危险类别码:R26/28
-
WGK Germany:3
-
危险品运输编号:UN 1544 6.1/PG 1
-
RTECS号:TJ2100000
-
包装等级:II
-
危险类别:6.1(a)
-
储存条件:本品应密封存放于阴凉避光处。
SDS
Section I.Chemical Product and Company Identification
Chemical Name Physostigmine free base
Portland OR
Synonym Pyrrolo[2,3-b]indol-5-ol, 1,2,3,3a,8,8a-hexahydro-
1,3a,8-trimethyl-, 5-(N-methylcarbamate), (3aS,8aR)-
(CA INDEX NAME); Eserine
Chemical Formula C15H21N3O2
57-47-6
CAS Number
Section II. Composition and Information on Ingredients
Chemical Name CAS Number Percent (%) TLV/PEL Toxicology Data
Physostigmine free base 57-47-6 Min. 98.0 Not available. Rat LD50 (oral) 4500 µg/kg
(HPLC,T) Mouse LD50 (oral) 3 mg/kg
Rabbit LD50 (oral)11200 µg/kg
Section III. Hazards Identification
Acute Health Effects Toxic if ingested or inhaled. Avoid prolonged contact with this material. Overexposure may result in serious illness or death.
Follow safe industrial hygiene practices and always wear proper protective equipment when handling this compound.
Chronic Health Effects CARCINOGENIC EFFECTS : Not available.
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Reproductive effects.
Rat TDLo Intraperitoneal 333 µg/kg, male 1 day prior to mating
TOXIC EFFECTS:
Effects on Fertility - Mating performance
Mouse TDLo Intraperitoneal 50 µg/kg, female 13 days of pregnancy
TOXIC EFFECTS:
Effects on Newborn - Biochemical and metabolic
Effects on Newborn - Behavioral
Repeated exposure to an highly toxic material may produce general deterioration of health by an accumulation in one or
many human organs.
Section IV. First Aid Measures
Check for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15
Eye Contact
minutes. Get medical attention.
In case of contact, immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing
Skin Contact
and shoes. Wash clothing before reuse. Thoroughly clean shoes before reuse. Get medical attention immediately.
Inhalation If the victim is not breathing, perform mouth-to-mouth resuscitation. Loosen tight clothing such as a collar, tie, belt or
waistband. If breathing is difficult, oxygen can be administered. Seek medical attention if respiration problems do not
improve.
Ingestion INDUCE VOMITING by sticking finger in throat. Lower the head so that the vomit will not reenter the mouth and throat.
Loosen tight clothing such as a collar, tie, belt or waistband. If the victim is not breathing, perform mouth-to-mouth
resuscitation. Examine the lips and mouth to ascertain whether the tissues are damaged, a possible indication that the toxic
material was ingested; the absence of such signs, however, is not conclusive.
Section V. Fire and Explosion Data
Not available.
May be combustible at high temperature. Auto-Ignition
Flammability
Flammable Limits Not available.
Flash Points 100°C (212°F)
Combustion Products These products are toxic carbon oxides (CO, CO2), nitrogen oxides (NO, NO2).
Fire Hazards
Not available.
Risks of explosion of the product in presence of mechanical impact: Not available.
Explosion Hazards
Risks of explosion of the product in presence of static discharge: Not available.
Continued on Next Page
Physostigmine free base
Fire Fighting Media
SMALL FIRE: Use DRY chemical powder.
LARGE FIRE: Use water spray, fog or foam. DO NOT use water jet.
and Instructions
Consult with local fire authorities before attempting large scale fire-fighting operations.
Section VI. Accidental Release Measures
Spill Cleanup Highly toxic material. Air and light sensitive material. Heat sensitive material.
Stop leak if without risk. DO NOT get water inside container. DO NOT touch spilled material. Use water spray to reduce
Instructions
vapors. Prevent entry into sewers, basements or confined areas; dike if needed. Eliminate all sources of ignition. Consult
federal, state, and/or local authorities for assistance on disposal.
Section VII. Handling and Storage
HIGHLY TOXIC. AIR AND LIGHT SENSITIVE. HEAT SENSITIVE MATERIAL. STORE UNDER INERT GAS. Keep locked
Handling and Storage
up. Keep away from heat. Mechanical exhaust required. When not in use, tightly seal the container and store in a dry, cool
Information
place. Avoid excessive heat and light. DO NOT ingest. Do not breathe dust. Wear suitable protective clothing. If ingested,
seek medical advice immediately and show the container or the label. Treat symptomatically and supportively.
Always store away from incompatible compounds such as oxidizing agents, metals, acids, alkalis (bases).
Section VIII. Exposure Controls/Personal Protection
Use process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below recommended
Engineering Controls
exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to airborne contaminants
below the exposure limit.
Splash goggles. Lab coat. Dust respirator. Boots. Gloves. A MSHA/NIOSH approved respirator must be used to avoid
Personal Protection
inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling this
product.
Exposure Limits Not available.
Section IX. Physical and Chemical Properties
Solid. (Off-white crystal.) Solubility
Physical state @ 20°C Very soluble in dichloromethane.
Soluble in ether, chloroform,
Not available. benzene, alcohol, oils.
Specific Gravity
Slightly soluble in water.
Molecular Weight 275.35 Partition Coefficient LOG Pow: 2.21
Boiling Point Not available. Not applicable.
Vapor Pressure
Melting Point 105°C (221°F) Vapor Density Not available.
Not available. Not available.
Refractive Index Volatility
Critical Temperature Not available. Odor Not available.
Not available. Not available.
Viscosity Taste
Section X. Stability and Reactivity Data
Stability
This material is stable if stored under proper conditions. (See Section VII for instructions)
Conditions of Instability Avoid excessive heat and light. Air and light sensitive. Store under inert gas.
Turns red on exposure to heat, light, air, and on contact with traces of metals.
Incompatibilities
Reactive with oxidizing agents, metals, acids, alkalis (bases).
Section XI. Toxicological Information
TJ2100000
RTECS Number
Routes of Exposure Eye Contact. Ingestion. Inhalation.
Rat LD50 (oral) 4500 µg/kg
Toxicity Data
Mouse LD50 (oral) 3 mg/kg
Rabbit LD50 (oral)11200 µg/kg
CARCINOGENIC EFFECTS : Not available.
Chronic Toxic Effects
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Reproductive effects.
Rat TDLo Intraperitoneal 333 µg/kg, male 1 day prior to mating
TOXIC EFFECTS:
Effects on Fertility - Mating performance
Mouse TDLo Intraperitoneal 50 µg/kg, female 13 days of pregnancy
TOXIC EFFECTS:
Effects on Newborn - Biochemical and metabolic
Effects on Newborn - Behavioral
Repeated exposure to an highly toxic material may produce general deterioration of health by an accumulation in one or many
human organs.
Toxic if ingested or inhaled. Avoid prolonged contact with this material. Overexposure may result in serious illness or death.
Acute Toxic Effects
Follow safe industrial hygiene practices and always wear proper protective equipment when handling this compound.
Continued on Next Page
Physostigmine free base
Section XII. Ecological Information
Ecotoxicity Not available.
Not available.
Environmental Fate
Section XIII. Disposal Considerations
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material with a
Waste Disposal
combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system. Observe all
federal, state and local regulations when disposing of the substance.
Section XIV. Transport Information
DOT Classification DOT CLASS 6.1: Toxic material.
PIN Number
Proper Shipping Name Alkaloids, solid, n.o.s.
I RQ = 100 (45.4)
Packing Group (PG)
DOT Pictograms
Section XV. Other Regulatory Information and Pictograms
TSCA Chemical Inventory This compound is ON the EPA Toxic Substances Control Act (TSCA) inventory list.
(EPA)
WHMIS Classification CLASS D-1A: Material causing immediate and serious toxic effects (VERY TOXIC).
On NDSL.
(Canada)
EINECS Number (EEC) 200-332-8
EEC Risk Statements R26/27/28- Very toxic by inhalation, in contact with skin and if swallowed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
毒扁豆碱亦称“依色林”,是一种吲哚类生物碱,化学式为C15H21N3O2。它存在于非洲西部的一种豆科植物——毒扁豆的种子中,含量约为0.1%。在乙醚中析出时呈片状结晶,熔点为105~106°C,该晶体不稳定,易变为熔点86~87°C的结晶。微溶于水,可溶于乙醇、苯、氯仿或脂肪油中。毒扁豆碱能抑制胆碱酯酶而引起机体中毒,是一种副交感神经兴奋剂,其中毒症状类似于神经性毒剂。口服中毒的人致死剂量为6~10毫克/人。
生物活性Physostigmine(Eserine)是一种可逆的乙酰胆碱酯酶(AChE)抑制剂,能够透过血脑屏障并刺激中枢胆碱能神经传递。它能够逆转阿尔茨海默病转基因小鼠的记忆缺陷,并且也是抗胆碱能中毒的解毒剂。
体内研究- 改善记忆功能:在异氟烷麻醉下,Physostigmine(0.1, 0.2 mg/kg)剂量大于等于0.2 mg/kg时可以延迟雄性Sprague-Dawley大鼠从麻醉中苏醒的时间。
- 转基因小鼠实验:Physostigmine (Eserine; 0.03-0.3 mg/kg;s.c.; 每日一次,持续6周) 能够在一定程度上改善heterozygous transgenic mice(Tg(+))的环境和提示记忆缺陷,使其更接近于非转基因小鼠的记忆表现。
毒扁豆碱为无色片状结晶,无特殊气味。遇光、热及微量金属时逐渐变红。该物质在碱性条件下不稳定,水解后生成毒扁豆酚碱,并进一步氧化生成红色的依色林红。其熔点为105-106°C,比旋光度[α]17D -76° (1.3%,氯仿),[α]25D -120°(苯)。易溶于二氯甲烷,可溶于氯仿(1:1)、乙醇(1:10)和乙醚(1:30),微溶于水。毒扁豆碱有毒性,成年鼠口服LD50为4.5毫克/公斤,小鼠为3毫克/公斤。
用途毒扁豆碱主要用于生化研究、药物合成中间体以及抗胆碱酯酶药的制备。它能够抑制体内胆碱酯酶的活力,是一种副交感神经兴奋剂,主要用于治疗青光眼,其效力较毛果芸香碱强而持久,可维持几个小时至几天。目前在中国还被用作中药麻醉的催醒药物。
生产方法毒扁豆碱存在于豆科植物——毒扁豆(Physostigma venenosum)的种子中。通过乙醇或丙酮萃取出生物碱后,将醇溶液或丙酮溶液与双氧水作用,在低温下蒸发未反应的生物碱,再用醚溶解生成毒扁豆碱N-氧化物。使用乙酸和锌粉还原可以恢复为毒扁豆碱。
安全信息 性质有毒物质
毒性分级剧毒
急性毒性大鼠口服LD50:4.5毫克/公斤;小鼠口服LD50: 3毫克/公斤
可燃性危险特性可燃,火场分解产生有毒氮氧化物气体
储运特性库房低温通风干燥;与食品原料分开存放
灭火剂上下游信息
反应信息
-
作为反应物:描述:physostigmine 以80%的产率得到参考文献:名称:YU, QIAN-SHENG;YEH, HERMAN J. C.;BROSSI, ARNOLD;FLIPPEN-ANDERSON, JUDITH +, J. NATUR. PROD., 52,(1989) N, C. 332-336摘要:DOI:
-
作为产物:描述:参考文献:名称:一种新颖高效的全合成(±)-毒扁豆碱摘要:Wittig烯烃化-Claisen重排方案在全合成(±)-毒扁豆碱中的应用。DOI:10.1016/j.tetlet.2009.03.012
文献信息
-
NOVEL GLUCOKINASE ACTIVATORS AND METHODS OF USING SAME申请人:Ryono Denis E.公开号:US20080009465A1公开(公告)日:2008-01-10Compounds are provided which are phosphonate and phosphinate activators and thus are useful in treating diabetes and related diseases and have the structure wherein is a heteroaryl ring; R 4 is —(CH 2 ) n -Z-(CH 2 ) m —PO(OR 7 )(OR 8 ), —(CH 2 ) n Z-(CH 2 ) m —PO(OR 7 )R g , —(CH 2 ) n -Z-(CH 2 ) m —OPO(OR 7 )R g , —(CH 2 ) n Z—(CH 2 ) m —OPO(R 9 )(R 10 ), or —(CH 2 ) n Z—(CH 2 ) m —PO(R 9 )(R 10 ); R 5 and R 6 are independently selected from H, alkyl and halogen; Y is R 7 (CH 2 ) s or is absent; and X, n, Z, m, R 4 , R 5 , R 6 , R 7 , and s are as defined herein; or a pharmaceutically acceptable salt thereof. A method for treating diabetes and related diseases employing the above compounds is also provided.提供了磷酸酯和磷酸酯激活剂,因此在治疗糖尿病和相关疾病方面非常有用,并具有以下结构: 其中 是杂环芳基环; R 4 为—(CH 2 ) n -Z-(CH 2 ) m —PO(OR 7 )(OR 8 )、—(CH 2 ) n Z-(CH 2 ) m —PO(OR 7 )R g 、—(CH 2 ) n -Z-(CH 2 ) m —OPO(OR 7 )R g 、—(CH 2 ) n Z—(CH 2 ) m —OPO(R 9 )(R 10) 或—(CH 2 ) n Z—(CH 2 ) m —PO(R 9 )(R 10) ; R 5 和R 6 分别选择自H、烷基和卤素; Y为R 7 (CH 2 ) s 或不存在;以及 X、n、Z、m、R 4 、R 5 、R 6 、R 7 和s如本文所定义;或其药用盐。 还提供了一种利用上述化合物治疗糖尿病和相关疾病的方法。
-
Novel Compounds申请人:Chhipa Laxmikant公开号:US20100168110A1公开(公告)日:2010-07-01The present invention discloses a novel thyroid like compounds of formula (I), wherein R 1 R 2 , R 3 , R 4 and Z are as defined in the specification, method for its preparation, composition containing such compounds and use of such compounds and composition as medicament. Further, compounds of formula (I) has significantly low binding affinity to thyroid receptors and thus considerably devoid of thyrotoxic effects. The invention also relates to the use of the compound of formula (I) for the preparation of a medicament for treating various disease conditions such as obesity, dyslipidemia, metabolic syndrome and co-morbidities associated with metabolic syndrome.
-
[EN] BIS-HETEROARYL DERIVATIVES AS MODULATORS OF PROTEIN AGGREGATION<br/>[FR] DÉRIVÉS BIS-HÉTÉROARYLIQUES EN TANT QUE MODULATEURS DE L'AGRÉGATION DES PROTÉINES申请人:NEUROPORE THERAPIES INC公开号:WO2017020010A1公开(公告)日:2017-02-02The present invention relates to certain bis-heteroaryl compounds, pharmaceutical compositions containing them, and methods of using them, including methods for preventing, reversing, slowing, or inhibiting protein aggregation, and methods of treating diseases that are associated with protein aggregation, including neurodegenerative diseases such as Parkinson's disease, Alzheimer's disease, Lewy body disease, Parkinson's disease with dementia, fronto- temporal dementia, Huntington's Disease, amyotrophic lateral sclerosis, and multiple system atrophy, and cancer including melanoma.
-
Amino-substituted heterocycles, compositions thereof, and methods of treatment therewith申请人:D'Sidocky Neil R.公开号:US20080242694A1公开(公告)日:2008-10-02Provided herein are Heterocyclic Compounds having the following structure: wherein R 1 , R 2 , X, Y and Z are as defined herein, compositions comprising an effective amount of a Heterocyclic Compound and methods for treating or preventing cancer, inflammatory conditions, immunological conditions, metabolic conditions and conditions treatable or preventable by inhibition of a kinase pathway comprising administering an effective amount of a Heterocyclic Compound to a patient in need thereof.
-
[EN] ALKOXY BIS-HETEROARYL DERIVATIVES AS MODULATORS OF PROTEIN AGGREGATION<br/>[FR] DÉRIVÉS BIS-HÉTÉROARYLIQUES D'ALCOXY UTILISÉS EN TANT QUE MODULATEURS DE L'AGRÉGATION DE PROTÉINES申请人:UCB BIOPHARMA SPRL公开号:WO2018138085A1公开(公告)日:2018-08-02The present invention relates to certain bis-heteroaryl compounds, pharmaceutical compositions containing them, and methods of using them, including methods for preventing, reversing, slowing, or inhibiting protein aggregation, and methods of treating diseases that are associated with protein aggregation, including neurodegenerative diseases such as Parkinson's disease, Alzheimer's disease, Lewy body disease, Parkinson's disease with dementia, fronto-temporal dementia, Huntington's Disease, amyotrophic lateral sclerosis, and multiple system atrophy, and cancer including melanoma.
表征谱图
-
氢谱1HNMR
-
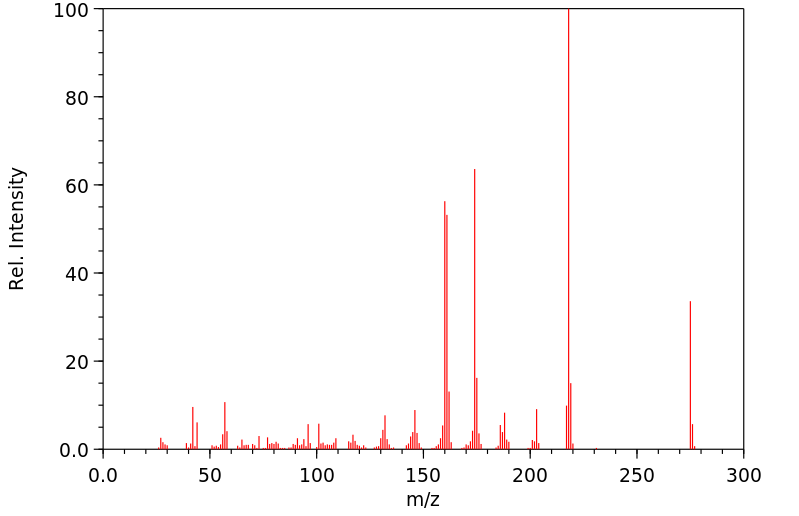
质谱MS
-
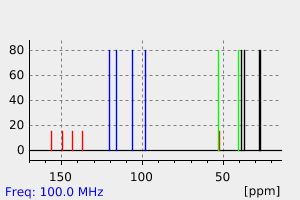
碳谱13CNMR
-
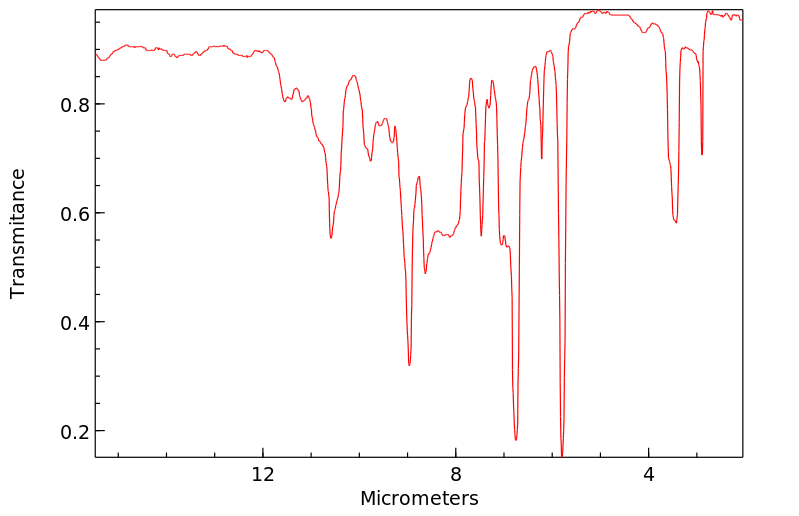
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息