5,6-二氨基-1,3-二甲基脲嘧啶 | 5440-00-6
中文名称
5,6-二氨基-1,3-二甲基脲嘧啶
中文别名
5,6-二氨基-1,3-二甲基尿苷水合物
英文名称
4,5-Diamino-1,3-dimethyluracil
英文别名
5,6-Diamino-1,3-dimethyluracil;5,6-diamino-1,3-dimethylpyrimidine-2,4(1H,3H)-dione;5,6-diamino-1,3-dimethylpyrimidine-2,4-dione
CAS
5440-00-6
化学式
C6H10N4O2
mdl
MFCD00006551
分子量
170.171
InChiKey
BGQNOPFTJROKJE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:210-214 °C (dec.)(lit.)
-
沸点:243.6±50.0℃ (760 Torr)
-
密度:1.350±0.06 g/cm3 (20 ºC 760 Torr)
-
闪点:101.1±30.1℃
-
溶解度:可溶于二甲基亚砜、甲醇
-
稳定性/保质期:
如果按照规定使用和储存,则不会分解,未曾发生过已知的危险。应避免与强氧化剂接触。
计算性质
-
辛醇/水分配系数(LogP):-0.5
-
重原子数:12
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:92.7
-
氢给体数:2
-
氢受体数:4
安全信息
-
WGK Germany:3
-
海关编码:2933599090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:应将药品存放在密闭、阴凉干燥处,并保持良好通风。同时,容器内应充入惰性气体以保证安全。
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product identifiers
Product name : 5,6-Diamino-1,3-dimethyluracil hydrate
CAS-No. : 5440-00-6
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Not a hazardous substance or mixture according to Regulation (EC) No 1272/2008
Not a hazardous substance or mixture according to EC-directives 67/548/EEC or 1999/45/EC.
Label elements
The product does not need to be labelled in accordance with EC directives or respective national laws.
Other hazards - none
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Synonyms : 5,6-Diamino-1,3-dimethyl-2,4(1H,3H)-pyrimidinedione
Formula : C6H10N4O2 · xH2O
Molecular Weight : 170,17 g/mol
Section 4. FIRST AID MEASURES
Description of first aid measures
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration.
In case of skin contact
Wash off with soap and plenty of water.
In case of eye contact
Flush eyes with water as a precaution.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water.
Most important symptoms and effects, both acute and delayed
Indication of any immediate medical attention and special treatment needed
no data available
Section 5. FIREFIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides, nitrogen oxides (NOx)
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Avoid dust formation. Avoid breathing vapors, mist or gas.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Sweep up and shovel. Keep in suitable, closed containers for disposal.
Reference to other sections
For disposal see section 13.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Provide appropriate exhaust ventilation at places where dust is formed.Normal measures for preventive fire
protection.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Store under inert gas.
Specific end uses
no data available
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
General industrial hygiene practice.
Personal protective equipment
Eye/face protection
Use equipment for eye protection tested and approved under appropriate government standards
such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
Choose body protection in relation to its type, to the concentration and amount of dangerous
substances, and to the specific work-place., The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Respiratory protection is not required. Where protection from nuisance levels of dusts are desired,
use type N95 (US) or type P1 (EN 143) dust masks. Use respirators and components tested and
approved under appropriate government standards such as NIOSH (US) or CEN (EU).
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
a) Appearance Form: solid
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing Melting point/range: 210 - 214 °C - dec.
point
f) Initial boiling point and no data available
boiling range
g) Flash point no data available
h) Evaporation rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility no data available
o) Partition coefficient: n- no data available
octanol/water
p) Autoignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
Section 10. STABILITY AND REACTIVITY
Reactivity
no data available
Chemical stability
no data available
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
Section 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Potential health effects
Inhalation
May be harmful if inhaled. May cause respiratory tract irritation.
Ingestion May be harmful if swallowed.
Skin May be harmful if absorbed through skin. May cause skin irritation.
Eyes May cause eye irritation.
Additional Information
RTECS: Not available
Section 12. ECOLOGICAL INFORMATION
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
no data available
Other adverse effects
no data available
Section 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
Section 15. REGULATORY INFORMATION
This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.
Safety, health and environmental regulations/legislation specific for the substance or mixture
no data available
Chemical Safety Assessment
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
用途:医药中间体。
生产方法:通过氰乙酸经过缩合、环合、亚硝化和还原步骤制得。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,3-二甲基-6-亚氨基-5-异亚硝基尿嘧啶 6-amino-1,3-dimethyl-5-nitroso-1H-pyrimidine-2,4-dione 6632-68-4 C6H8N4O3 184.155 6-氨基-5-甲酰氨基-1,3-二甲基尿嘧啶 1,3-dimethyl-4-amino-5-(formylamino)uracil 7597-60-6 C7H10N4O3 198.181 6-氨基-1,3-二甲基-5-亚硝基尿嘧啶 6-amino-1,3-dimethyl-5-nitro-1H-pyrimidine-2,4-dione 3346-61-0 C6H8N4O4 200.154 —— 5-acetylamino-6-amino-1,3-dimethyl-1H-pyrimidine-2,4-dione 10184-41-5 C8H12N4O3 212.208 1,3-二甲基-6-氨基脲嘧啶 6-Amino-1,3-dimethylbarbituric acid 6642-31-5 C6H9N3O2 155.156 —— 1,3-dimethyl-5-nitro-6-hydrazinouracil 906428-70-4 C6H9N5O4 215.169 —— 5-acetylamino-6-amino-1-methyl-1H-pyrimidine-2,4-dione 10184-42-6 C7H10N4O3 198.181 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 6-氨基-5-甲酰氨基-1,3-二甲基尿嘧啶 1,3-dimethyl-4-amino-5-(formylamino)uracil 7597-60-6 C7H10N4O3 198.181 —— N-(6-amino-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydro-pyrimidin-5-yl)-glycine-nitrile 7468-65-7 C8H11N5O2 209.208 —— 5-acetylamino-6-amino-1,3-dimethyl-1H-pyrimidine-2,4-dione 10184-41-5 C8H12N4O3 212.208 —— 6-amino-5-(benzylideneamino)-1,3-dimethyluracil 60228-89-9 C13H14N4O2 258.28 —— 6-amino-5-(benzylideneamino)-1,3-dimethylpyrimidine-2,4-dione 60228-89-9 C13H14N4O2 258.28 —— 6-amino-1,3-dimethyl-5-[(E)-3-phenyl-prop-2-en-(E)-ylideneamino]-1H-pyrimidine-2,4-dione 1198748-32-1 C15H16N4O2 284.318 —— N-(6-amino-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)-2-hydroxyacetamide 6743-05-1 C8H12N4O4 228.208 —— 6-amino-5-(4-chloro-benzylideneamino)-1,3-dimethyl-1H-pyrimidine-2,4-dione 13784-07-1 C13H13ClN4O2 292.725 —— 6-amino-5-[(4-chlorophenyl)methylideneamino]-1,3-dimethylpyrimidine-2,4-dione 13784-07-1 C13H13ClN4O2 292.725 —— 6-amino-5-{[1-(3-chlorophenyl)-meth-(E)-ylidene]-amino}-1,3-dimethyl-1H-pyrimidine-2,4-dione 1198748-31-0 C13H13ClN4O2 292.725 —— 6-amino-5-[(E)-3-(3-chloro-phenyl)-prop-2-en-(E)-ylideneamino]-1,3-dimethyl-1H-pyrimidine-2,4-dione 1198748-33-2 C15H15ClN4O2 318.763 —— 6-amino-5-((E)-2-butenoylamino)-1,3-dimethyluracil 157399-91-2 C10H14N4O3 238.246 —— 6-Amino-5-(4-methoxybenzylideneamino)-1,3-dimethyluracil 60333-34-8 C14H16N4O3 288.306 —— 6-amino-5-(3-butenoylamino)-1,3-dimethyluracil 99171-67-2 C10H14N4O3 238.246 —— cyano-acetic acid-(6-amino-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydro-pyrimidin-5-ylamide) 5463-54-7 C9H11N5O3 237.218 (6-氨基-1,2,3,4-四氢-1,3-二甲基-2,4-二羰基-5-嘧啶基)氨基甲酸乙酯 (6-amino-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydro-pyrimidin-5-yl)-carbamic acid ethyl ester 49810-21-1 C9H14N4O4 242.235 —— 6-amino-1,3-dimethyl-5-(1-methyl-3-oxo-but-1-enylamino)-1H-pyrimidine-2,4-dione 57196-68-6 C11H16N4O3 252.273 —— 6-amino-1,3-dimethyl-5-[(pyridin-2-ylmethylidene)amino]pyrimidine-2,4(1H,3H)-dione 64232-66-2 C12H13N5O2 259.268 N-(6-氨基-1,3-二甲基-2,4-二氧代-1,2,3,4-四氢-5-嘧啶基)-3-甲氧基丙烷酰胺 N-(6-Amino-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)-3-methoxypropanamide 90749-77-2 C10H16N4O4 256.261 —— 6-amino-1,3-dimethyl-5-heptanoylaminouracil —— C13H22N4O3 282.343 —— 6-amino-1,3-dimethyl-5-nonanoylaminouracil —— C15H26N4O3 310.396 —— N-(4-amino-1,3-dimethyl-2,6-dioxopyrimidin-5-yl)-4-methoxybutanamide 90749-78-3 C11H18N4O4 270.288 —— 6-amino-1,3-dimethyl-5-[[(2S,3S,4R)-2,3,4,5-tetrahydroxypentylidene]amino]pyrimidine-2,4-dione 88154-61-4 C11H18N4O6 302.287 —— 6-amino-5-[[4-(dimethylamino)phenyl]methylideneamino]-1,3-dimethylpyrimidine-2,4-dione 20886-61-7 C15H19N5O2 301.348 N-(6-氨基-1,3-二甲基-2,4-二氧代-1,2,3,4-四氢-5-嘧啶基)-2-羟基丙酰胺 N-(6-amino-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydro-pyrimidin-5-yl)-lactamide 100144-13-6 C9H14N4O4 242.235 5-[(6-氨基-1,3-二甲基-2,4-二氧代-1,2,3,4-四氢-5-嘧啶基)氨基]-5-氧代戊酸 5-(6-amino-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-ylamino)-5-oxopentanoic acid 109418-98-6 C11H16N4O5 284.272 —— 6-amino-1,3-dimethyl-5-N-D-glucosylideneiminouracil 58045-65-1 C12H20N4O7 332.313 (5CI)-6-氨基-5-(2-苯胺乙酰氨基)-1,3-二甲基-尿嘧啶 N-phenyl-glycin-(6-amino-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydro-1H-pyrimidin-5-ylamide) 857474-92-1 C14H17N5O3 303.321 二乙基[[(3-氯-4-氟苯基)氨基]亚甲基]丙二酸酯 6-amino-5-benzoylamino-1,3-dimethyl-1H-pyrimidine-2,4-dione 964-04-5 C13H14N4O3 274.279 —— DL-glyceric acid-(6-amino-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydro-pyrimidin-5-ylamide) 108630-84-8 C9H14N4O5 258.234 —— N-(6-amino-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)cinnamamide —— C15H16N4O3 300.317 —— 6-amino-5-[{3-(2-dimethylaminoethoxy)benzylidene}amino]-1,3-dimethyluracil 1155224-38-6 C17H23N5O3 345.401 —— 6-amino-5-[{3-(2-diethylaminoethoxy)benzylidene}amino]-1,3-dimethyluracil 1155224-39-7 C19H27N5O3 373.455 —— 4-amino-5-<(2-oxopyrrolidinyl-1)acetamido>-1,3-dimethyluracil monohydrate 132351-15-6 C12H17N5O4 295.298 —— N-(4-amino-1,3-dimethyl-2,6-dioxopyrimidin-5-yl)-4-methoxybenzamide 166115-69-1 C14H16N4O4 304.305 —— (E)-N-(4-amino-1,3-dimethyl-2,6-dioxopyrimidin-5-yl)-3-(4-methoxyphenyl)prop-2-enamide 1026008-03-6 C16H18N4O4 330.343 —— (E)-N-(4-amino-1,3-dimethyl-2,6-dioxopyrimidin-5-yl)-3-(3-chlorophenyl)prop-2-enamide 1026497-99-3 C15H15ClN4O3 334.762 —— 6-amino-5-{[(3-cyclopentyloxy)benzylidene]amino}-1,3-dimethyluracil 1262151-06-3 C18H22N4O3 342.398 —— (E)-N-(4-amino-1,3-dimethyl-2,6-dioxopyrimidin-5-yl)-3-(3-fluorophenyl)prop-2-enamide 1025813-84-6 C15H15FN4O3 318.308 —— 6-amino-5-<<4-(trifluoromethyl)benzoyl>amino>-1,3-dimethyluracil 149981-35-1 C14H13F3N4O3 342.277 —— 6-amino-5-[{3-(2-morpholin-4-ylethoxy)benzylidene}amino]-1,3-dimethyluracil 1155224-41-1 C19H25N5O4 387.439 —— 6-amino-5-[{3-(2-pyrrolidin-1-ylethoxy)benzylidene}amino]-1,3-dimethyluracil 1155224-42-2 C19H25N5O3 371.439 4-((2-呋喃甲基)硫代)-2-戊酮 6-amino-1,3-dimethyl-5-perfluoropropanoylaminouracil 288391-11-7 C9H9F5N4O3 316.187 —— (E)-N-(4-amino-1,3-dimethyl-2,6-dioxopyrimidin-5-yl)-3-[4-(dimethylamino)phenyl]prop-2-enamide 1025972-39-7 C17H21N5O3 343.385 —— 6-amino-5-[{3-(2-piperidin-1-ylethoxy)benzylidene}amino]-1,3-dimethyluracil 1155224-40-0 C20H27N5O3 385.466 —— (E)-N-(4-amino-1,3-dimethyl-2,6-dioxopyrimidin-5-yl)-3-(3,5-difluorophenyl)prop-2-enamide 1026797-30-7 C15H14F2N4O3 336.298 —— 6-amino-1,3-dimethyl-5-(2-aminobenzamido)uracil 18830-59-6 C13H15N5O3 289.294 —— N-(4-amino-1,3-dimethyl-2,6-dioxopyrimidin-5-yl)-2-phenoxypropanamide —— C15H18N4O4 318.332 —— sulfanilic acid-(6-amino-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydro-pyrimidin-5-ylamide) 53385-40-3 C12H15N5O4S 325.348 —— (E)-N-(4-amino-1,3-dimethyl-2,6-dioxopyrimidin-5-yl)-3-(3-methoxyphenyl)prop-2-enamide 1026503-62-7 C16H18N4O4 330.343 N-(6-氨基-1,3-二甲基-2,4-二氧代-1,2,3,4-四氢-5-嘧啶基)-4-硝基苯甲酰胺 N-(6-Amino-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)-4-nitrobenzamide 249929-77-9 C13H13N5O5 319.277 —— 6-amino-5-[{4-(2-dimethylaminoethoxy)-3-methoxybenzylidene}amino]-1,3-dimethyluracil 1155224-14-8 C18H25N5O4 375.428 —— 6-amino-5-[{3-(2-dimethylaminoethoxy)-4-methoxybenzylidene}amino]-1,3-dimethyluracil 1155224-26-2 C18H25N5O4 375.428 —— thiophene-2-carboxylic acid 6-amino-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydro-pyrimidin-5-ylamide 26256-61-1 C11H12N4O3S 280.307 —— Pyrimidin-2,4-dione, 6-amino-5-[[4-[4-(methylphenyl)aminocarbonyl]methoxy]benzimidoyl]-1,3-dipropyl- —— C22H23N5O4 421.456 —— N-(4-amino-1,3-dimethyl-2,6-dioxopyrimidin-5-yl)-3-methoxybenzamide 166115-68-0 C14H16N4O4 304.305 —— 6-amino-5-[{4-(2-diethylaminoethoxy)-3-methoxybenzylidene}amino]-1,3-dimethyluracil 1155224-15-9 C20H29N5O4 403.481 —— 6-amino-5-[{3-(2-diethylaminoethoxy)-4-methoxybenzylidene}amino]-1,3-dimethyluracil 1155224-27-3 C20H29N5O4 403.481 —— 1,3-dimethyl-6-amino-5-(p-sulfobenzamido)uracil 94781-74-5 C13H14N4O6S 354.343 —— (E)-N-(4-amino-1,3-dimethyl-2,6-dioxopyrimidin-5-yl)-3-[3-(trifluoromethyl)phenyl]prop-2-enamide 1026284-54-7 C16H15F3N4O3 368.315 —— N-(4-amino-1,3-dimethyl-2,6-dioxopyrimidin-5-yl)tricyclo[3.3.1.03,7]nonane-3-carboxamide 136199-25-2 C16H22N4O3 318.376 —— (E)-N-(4-amino-1,3-dimethyl-2,6-dioxopyrimidin-5-yl)-3-(3,5-dimethoxyphenyl)prop-2-enamide 1027389-46-3 C17H20N4O5 360.37 —— (E)-N-(4-amino-1,3-dimethyl-2,6-dioxopyrimidin-5-yl)-3-(2-methoxyphenyl)prop-2-enamide 1026296-36-5 C16H18N4O4 330.343 —— N-(4-amino-1,3-dimethyl-2,6-dioxopyrimidin-5-yl)-2-hydroxy-2-phenylacetamide 314774-00-0 C14H16N4O4 304.305 —— 6-amino-5-[{3-methoxy-4-(2-morpholin-4-ylethoxy)benzylidene}amino]-1,3-dimethyluracil 1155224-18-2 C20H27N5O5 417.465 —— 5,6-diacetylamino-1,3-dimethyluracil 64589-42-0 C10H14N4O4 254.246 —— (E)-N-(4-amino-1,3-dimethyl-2,6-dioxopyrimidin-5-yl)-3-(3-nitrophenyl)prop-2-enamide 1026098-13-4 C15H15N5O5 345.315 —— 6-amino-5-[{4-methoxy-3-(2-morpholin-4-ylethoxy)benzylidene}amino]-1,3-dimethyluracil 1155224-30-8 C20H27N5O5 417.465 —— 6-amino-5-[{3-methoxy-4-(2-pyrrolidin-1-ylethoxy)benzylidene}amino]-1,3-dimethyluracil 1155224-19-3 C20H27N5O4 401.465 —— 6-amino-5-{[(4-cyclopentyloxy)-3-methoxybenzylidene]amino}-1,3-dimethyluracil 1262151-00-7 C19H24N4O4 372.424 —— 6-amino-1,3-dimethyl-5-(N-acetyl-DL-methionyl)aminouracil —— C13H21N5O4S 343.407 —— 6-amino-5-[{4-methoxy-3-(2-pyrrolidin-1-ylethoxy)benzylidene}amino]-1,3-dimethyluracil 1155224-32-0 C20H27N5O4 401.465 —— 6-amino-5-[2-(4-isobutylphenyl)propanoyl]amino-1,3-dimethyl-1H-pyrimidine-2,6-dione 872051-97-3 C19H26N4O3 358.44 —— 6-amino-5-{[(3-cyclopentyloxy)-4-methoxybenzylidene]amino}-1,3-dimethyluracil 1262151-02-9 C19H24N4O4 372.424 —— 6-amino-5-[{3-methoxy-4-(2-piperidin-1-ylethoxy)benzylidene}amino]-1,3-dimethyluracil 1155224-16-0 C21H29N5O4 415.492 —— N-(4-amino-1,3-dimethyl-2,6-dioxopyrimidin-5-yl)-2-phenoxybutanamide —— C16H20N4O4 332.359 —— (E)-N-(4-amino-1,3-dimethyl-2,6-dioxopyrimidin-5-yl)-3-(3,4-dimethoxyphenyl)prop-2-enamide 199680-98-3 C17H20N4O5 360.37 —— 6-amino-5-[{4-methoxy-3-(2-piperidin-1-ylethoxy)benzylidene}amino]-1,3-dimethyluracil 1155224-29-5 C21H29N5O4 415.492 N-(6-氨基-1,3-二甲基-2,4-二氧代-1,2,3,4-四氢-5-嘧啶基)-2-甲氧基苯甲酰胺 N-(4-amino-1,3-dimethyl-2,6-dioxopyrimidin-5-yl)-2-methoxybenzamide 166115-65-7 C14H16N4O4 304.305 —— N-[(4-amino-1,3-dimethyl-2,6-dioxopyrimidin-5-yl)carbamothioyl]furan-2-carboxamide 87874-14-4 C12H13N5O4S 323.332 —— (4-{[6-amino-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydro-pyrimidin-5-ylcarbamoyl]-methyl}-phenyl)-carbamic acid tert-butyl ester 748148-97-2 C19H25N5O5 403.438 7-氨基-1,3-二甲基-5,6-二氢蝶啶-2,4-二酮 7-Amino-5,6-dihydro-1,3-dimethyllumazin 7464-72-4 C8H11N5O2 209.208 —— N-(6-Amino-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)-3,4-dimethoxybenzamide 166115-70-4 C15H18N4O5 334.332 —— 3-[3,4,5-Trimethoxyphenyl]propenoic amide, N-[6-amino-3,5- 959030-60-5 C18H22N4O6 390.396 —— N-(4-amino-1,3-dimethyl-2,6-dioxopyrimidin-5-yl)-4-(diethoxyphosphorylmethyl)benzamide 166115-75-9 C18H25N4O6P 424.393 —— 6-amino-5-[2-(6-methoxy-2-naphthyl)propanoyl]amino-1,3-dimethyl-1H-pyrimidine-2,6-dione 872051-98-4 C20H22N4O4 382.419 —— β-(4-Amino-1,3-dimethyluracil-5-amino)-chalkon 57196-69-7 C21H20N4O3 376.415 —— 6-amino-1,3-dimethyl-5-perfluorobutanoylaminouracil —— C10H9F7N4O3 366.195 —— (E)-N-(4-amino-1,3-dimethyl-2,6-dioxopyrimidin-5-yl)-3-(2,3-dimethoxyphenyl)prop-2-enamide 1026126-04-4 C17H20N4O5 360.37 —— 6-amino-5-(3,5-di-tert-butyl-4-hydroxyphenylcarboxamido)-1,3-dimethyl-2,4-(1H,3H)-pyrimidinedione 595558-79-5 C21H30N4O4 402.494 —— 5,7-Diketo-4,6-dimethyl-2-phenyl-1H-2,3,4,5,6,7-hexahydropyrimido<4,5-d><2,1,3>=boradiazol 14320-99-1 C12H13BN4O2 256.072 —— 5-acetylamino-6-(acetyl-methyl-amino)-1,3-dimethyl-1H-pyrimidine-2,4-dione 109962-50-7 C11H16N4O4 268.272 —— (E)-N-(4-amino-1,3-dimethyl-2,6-dioxopyrimidin-5-yl)-3-(2,3,4-trimethoxyphenyl)prop-2-enamide 1028268-39-4 C18H22N4O6 390.396 —— 6-amino-1,3-dimethyl-5-[4-[[p-nitrophenoxy]sulfonyl]benzamido]uracil 666715-91-9 C19H17N5O8S 475.439 —— 5-amino-6-mercapto-1,3-dimethyluracil —— C6H9N3O2S 187.222 (6CI)-1,3-二甲基-5,6-双(N-甲基乙酰氨基)-尿嘧啶 N,N'-(1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydro-pyrimidine-4,5-diyl)-N,N'-dimethyl-bis-acetamide 105143-41-7 C12H18N4O4 282.299 —— 6-amino-1,3-dimethyl-5-perfluorononanoylaminouracil —— C15H9F17N4O3 616.234 —— 6-amino-1,3-dimethyl-5-[4-[[m-nitrophenoxy]sulfonyl]benzamido]uracil 666715-87-3 C19H17N5O8S 475.439 —— N1-(4-amino-1,3-dimethyl-uracil-5-yl)-2-[6-phenyl-pyridazin-3(2H)-one-2-yl]-acetamide 346407-20-3 C18H18N6O4 382.379 —— 6-amino-5-{[(4-cyclopentyloxy)-3-methoxy-2-nitrobenzylidene]amino}-1,3-dimethyluracil 1262151-14-3 C19H23N5O6 417.422 - 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
反应信息
-
作为反应物:描述:参考文献:名称:一种1,3,7,9-四甲基尿酸的全合成方法摘要:本发明涉及一种1,3,7,9‑四甲基尿酸的全合成方法,包括以6‑氨基‑1,3‑二甲基尿嘧啶为原料,经过亚硝基化反应得到中间体6‑氨基‑1,3‑二甲基‑5‑亚硝基尿嘧啶;6‑氨基‑1,3‑二甲基‑5‑亚硝基尿嘧啶经还原反应得到中间体1,3‑二甲基‑5,6‑二氨基尿嘧啶;1,3‑二甲基‑5,6‑二氨基尿嘧啶进行环合反应生成中间体1,3‑二甲基尿酸;1,3‑二甲基尿酸和甲基化试剂进行反应得到产物。采用本发明方法来制备四甲基尿酸,可在工业应用上大量合成,不受原料对其的约束;转化率高,产率高,合成方便,后处理较简单,适合大规模生产,能够广泛的推广应用;无须在高压条件下进行,对设备要求低,同时减少了危险的发生率;利用重结晶放置制备目标产物,产物纯度高。公开号:CN106046004B
-
作为产物:描述:参考文献:名称:Bredereck; Edenhofer, Chemische Berichte, 1955, vol. 88, p. 1306,1309摘要:DOI:
-
作为试剂:描述:1,10-邻二氮杂菲-5,6-二酮 在 5,6-二氨基-1,3-二甲基脲嘧啶 、 溶剂黄146 作用下, 以 乙醇 为溶剂, 以21%的产率得到1,10-phenanthroline-5,6-diol hydrochloride参考文献:名称:5,6-Dihydroxy-1,10-phenanthrolinium-1,10-ium dichloride摘要:5,6- 二羟基-1,10-菲罗啉是 1,10-菲罗啉-5,6-二酮和 5,6- 二氨基-1,3-二甲基脲嘧啶缩合反应的副产物,通过乙腈蒸发扩散到乙醇/HCl 溶液中分离出二盐酸盐 (C12H8N2O2-2HCl-0.5CH3CN)。该化合物在三linic 空间群 $$ P\overline{1} $$ 中结晶,a = 5.8334(1) 埃,b = 9.8041(2) 埃,c = 11.9895(2) 埃,α = 81.511(1)°,β = 76.395(1)°,γ = 81.429(1)°,V = 654.48(2) Å3,Z = 2,R[F 2 > 2σ(F 2)] = 0.028,wR(F 2) = 0.078。两种 N-H+-Cl- 相互作用(2.18(3) 埃、2.13(2) 埃)和两种 O-H-Cl- 相互作用(2.11(3) 埃、2.20(3) 埃)稳定了晶体堆积。菲罗啉环的π-堆积导致平面间距为 3.431(5) 埃,菲罗啉环的中心点彼此相距约 6 埃。该双质子化物种的结果与 Lin 等人(Acta Cryst E65:o2367, 2009)报告的单质子化形式的结果进行了比较,从而揭示了使用 -OH/ 菲罗啉环的相对取向作为该配体的金属配合物中金属-配体相互作用的定性诊断的可能性(Larsson 和 Ohrstrom,发表于 Inorg Chim Acta 357:657, 2004;Guan 等人,发表于 Acta Cryst C64:m311, 2008)。本文讨论了 5,6-二羟基-1,10-菲罗啉的二质子化形式(5,6-二羟基-1,10-菲罗啉鎓-1,10-二氯化物)的晶体结构,并将其与文献中报道的单质子化形式以及与锰(II)和钴(III)络合的形式进行了比较。DOI:10.1007/s10870-011-0003-0
文献信息
-
Synthesis and Regioselective N- and O-Alkylation of 1<i>H</i>- or 3<i>H</i>-[1,2,3]Triazolo[4,5-<i>d</i>]pyrimidine-5,7(4<i>H</i>,6<i>H</i>)-diones (8-Azaxanthines) and Transformation of Their 3-Alkyl Derivatives into 1-Alkyl Isomers作者:Tomohisa Nagamatsu、Rafiqul IslamDOI:10.1055/s-2006-950337日期:——alkylating agents, for example alkyl halides and dimethyl sulfate, were employed in aprotic Solvents Under a variety of conditions for the alkylation of mono- and disubstituted 1H- or 3H-[1,2,3]triazolo[4,5-d]pyrimidine-5,7(4H,6H)-diones, which were prepared by cyclization of the appropriate 5,6-diaminouracils with nitrous acid. The alkylation on the triazole ring in the presence of anhydrous potassium carbonate
-
Adenosine A1 antagonists. 2. Structure-activity relationships on diuretic activities and protective effects against acute renal failure作者:Fumio Suzuki、Junichi Shimada、Hideaki Mizumoto、Akira Karasawa、Kazuhiro Kubo、Hiromi Nonaka、Akio Ishii、Takashi KawakitaDOI:10.1021/jm00094a022日期:1992.8Diuretic activities of xanthine or nonxanthine adenosine antagonists and their ameliorative effects against glycerol-induced acute renal failure in rats were investigated in order to clarify the physiological and pathological function of adenosine receptors in the kidney. Diuretic and natriuretic activities of a variety of adenosine antagonists clarified systematically for the first time that the blockade为了阐明肾脏中腺苷受体的生理和病理功能,研究了黄嘌呤或非黄嘌呤腺苷拮抗剂的利尿作用及其对甘油诱导的急性肾衰竭的缓解作用。各种腺苷拮抗剂的利尿和利尿作用首次系统性地阐明,在钠和水的排泄中,A1受体的阻滞比A2受体的阻滞更重要,并支持内源性肾内腺苷水平直接增强肾小管钠吸收的假说。 。急性肾衰竭中8取代的黄嘌呤的构效关系研究表明,腺苷A1受体的激活是发展这种肾衰竭的重要因素。一系列8-(3-去甲金刚烷基)黄嘌呤表现出极强的利尿和利尿活性(24; 2.5微克/千克,po,治疗大鼠尿排泄值与对照大鼠尿排泄值之比= 1.69,该比率(治疗大鼠的Na + / K +相对于对照组大鼠的Na + / K + = 1.76)和抗甘油诱导的急性肾衰竭的有效改善作用(24; 10微克/ kg,腹腔注射,抑制55%)。通过对结构-活性关系的详细研究,我们可以推测,肾脏和大脑之间可能存在腺苷A1受体的一些组织差异,并
-
Immunosuppressive effects of 8-substituted xanthine derivatives
-
Immunosuppressive effects of pteridine derivatives申请人:——公开号:US20030236255A1公开(公告)日:2003-12-25Novel poly-substituted pteridinediones (lumazines), and mono- or polysubstituted 2-thiolumazines, 4-thiolumazines or 2,4-dithiolumazines, having disclosed substituents in positions 1, 3, 6 and 7 of the pteridine ring, and pharmaceutically acceptable salts thereof, are useful as biologically active ingredients in preparing pharmaceutical compositions especially for the treatment or prevention of a CNS disorder, a cell proliferative disorder, a viral infection, an immune or auto-immune disorder or a transplant rejection. Combinations of the pteridine derivatives of the invention with an immunosuppressant or immunomodulator drug, an antineoplastic drug or an antiviral agent, providing potential synergistic effects, are also disclosed.
-
Synthesis of Deuterium Labeled Standards of 1-Benzylpiperazine, Fenetylline, Nicocodeine and Nicomorphine作者:Zong-Yi You、Yi-Jing Chen、Yu-Yun Wang、Chinpiao ChenDOI:10.1002/jccs.200800099日期:2008.6analogs (designer drugs) with a large variety of structures have reached illegal markets, making their identification difficult. This work studies the synthesis of BZP-d 7 , fenetylline-d 4 , nicocodeine-d 4 and nicomorphine-d 8 , as internal standards for use in gas chromatography-mass spectrometry (GC-MS) analysis for identification of these controlled substances.
表征谱图
-
氢谱1HNMR
-
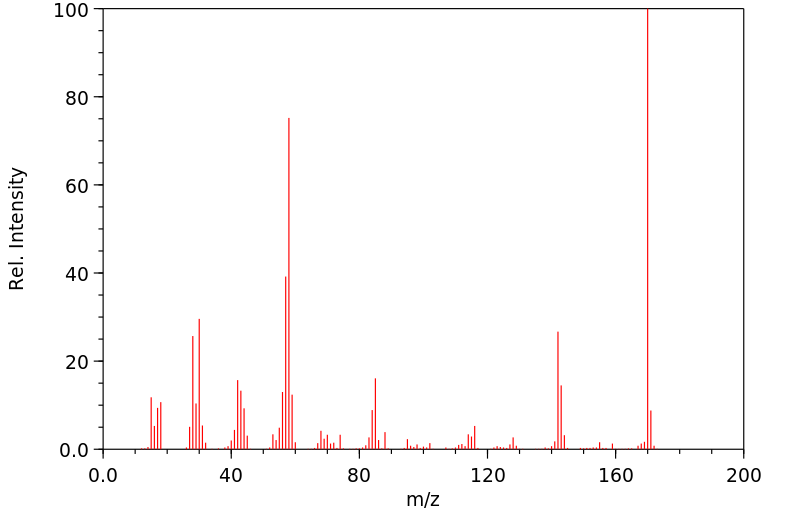
质谱MS
-
碳谱13CNMR
-
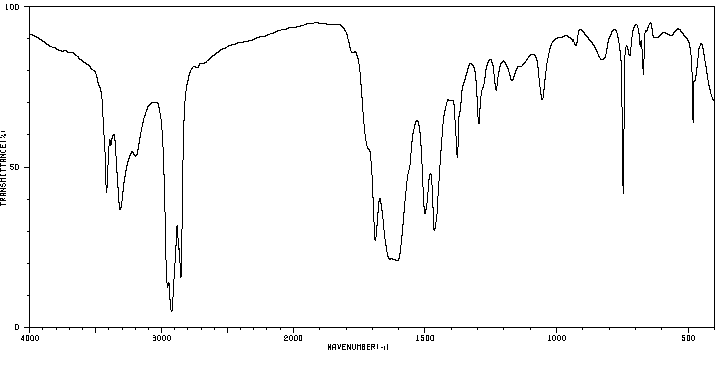
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-3-(2-(二氟甲基)吡啶-4-基)-7-氟-3-(3-(嘧啶-5-基)苯基)-3H-异吲哚-1-胺
(6-羟基嘧啶-4-基)乙酸
(4,5-二甲氧基-1,2,3,6-四氢哒嗪)
鲁匹替丁
马西替坦杂质7
马西替坦杂质4
马西替坦杂质
马西替坦原料药杂质D
马西替坦原料药杂质B
马西替坦
顺式-4-{[5-溴-2-(2,5-二甲基-1H-吡咯-1-基)-6-甲基嘧啶-4-基]氨基}环己醇
非沙比妥
非巴氨酯
非尼啶醇
青鲜素钾盐
雷特格韦钾盐
雷特格韦相关化合物E(USP)
雷特格韦杂质8
雷特格韦EP杂质H
雷特格韦-RT9
雷特格韦
阿西莫司杂质3
阿西莫司
阿脲四水合物
阿脲一水合物
阿维霉素
阿米美啶
阿米洛利
阿米妥钠
阿洛巴比妥
阿普瑞西他滨
阿普比妥
阿巴卡韦相关化合物B(USP)
阿卡明
阿伐那非杂质V
阿伐那非杂质1
阿伐那非杂质
阿伐那非中间体
阿伐那非
铂(2+)二氯化6-甲基-1,3-二{2-[(2-甲基丙基)硫烷基]乙基}嘧啶-2,4(1H,3H)-二酮(1:1)
钴1,2,3,6-四氢-2,6-二氧代嘧啶-4-羧酸酯(1:2)
钠5-烯丙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-乙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-(2-溴丙-2-烯基)-5-丁烷-2-基-4,6-二氧代-1H-嘧啶-2-醇
醌肟腙
酒石酸噻吩嘧啶
那可比妥
辛基2,6-二氧代-1,2,3,6-四氢-4-嘧啶羧酸酯
赛乐西帕杂质3
赛乐西帕KSM3