5-羟基尿嘧啶 | 20636-41-3
中文名称
5-羟基尿嘧啶
中文别名
2,4,5-三羟基嘧啶
英文名称
2,4,5-trihydroxypyrimidine
英文别名
5-hydroxypyrimidine-2,4(1H,3H)-dione;isobarbituric acid;trihydroxypyrimidine;dihydro-pyrimidine-2,4,5-trione;5-hydroxy-uracil;5-hydroxy-2,4(1H,3H)-pyrimidinedione;5-Hydroxyuracil;5-hydroxy-1H-pyrimidine-2,4-dione
CAS
20636-41-3
化学式
C4H4N2O3
mdl
MFCD11615701
分子量
128.087
InChiKey
OFJNVANOCZHTMW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:>300 °C (dec.)(lit.)
-
密度:1.605±0.06 g/cm3(Predicted)
-
溶解度:可溶于水基(轻微)、DMSO(轻微、超声处理)、甲醇(轻微)
计算性质
-
辛醇/水分配系数(LogP):-1.1
-
重原子数:9
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:78.4
-
氢给体数:3
-
氢受体数:3
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2933599090
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (2,4-二氧代-1H-嘧啶-5-基)乙酸酯 5-acetoxy-1H-pyrimidine-2,4-dione 3328-20-9 C6H6N2O4 170.125
反应信息
-
作为反应物:描述:参考文献:名称:Biltz; Paetzold, Justus Liebigs Annalen der Chemie, 1927, vol. 452, p. 79摘要:DOI:
-
作为产物:描述:尿嘧啶 在 sodium hydroxide 、 sodium persulfate 作用下, 以70%的产率得到5-羟基尿嘧啶参考文献:名称:异舒拉米和异巴比妥酸的简单合成摘要:尿嘧啶和 6-氨基尿嘧啶的过二硫酸盐氧化随后水解导致异巴比妥酸和异尿嘧啶的有效合成。DOI:10.1039/a800135i
文献信息
-
Design and synthesis of uracil urea derivatives as potent and selective fatty acid amide hydrolase inhibitors作者:Yan Qiu、Jie Ren、Hongwei Ke、Yang Zhang、Qi Gao、Longhe Yang、Canzhong Lu、Yuhang LiDOI:10.1039/c7ra02237a日期:——picomolar FAAH inhibitors (4c, IC50 = 0.3 ± 0.05 nM; 4d, IC50 = 0.8 ± 0.1 nM) were developed. Compound 4c inhibited FAAH in a rapid, selective, noncompetitive, and irreversible pattern. This study provides several highly potent and selective FAAH inhibitors and an optimized chemical scaffold for the development of FAAH inhibitors. We anticipate that these FAAH inhibitors will enable new possibilities in脂肪酸酰胺水解酶(FAAH)是参与内源性大麻素(尤其是anandamide)生物降解的关键酶之一。FAAH的药理学阻断作用可恢复内源性大麻素的水平,从而在治疗炎症,抑郁和多发性硬化症方面提供治疗益处。在这项研究中,设计并合成了一系列尿嘧啶脲衍生物作为FAAH抑制剂。N-己基-2,4-二氧代-3,4-二氢嘧啶-1(2 H)-羧酰胺(1a的C5位置和侧链的结构修饰)导致FAAH抑制剂具有更高的效能和选择性。结构-活性关系(SAR)研究表明,C5吸电子取代基优选具有最佳效能,但不具有选择性,而用苯基烷基基团或联苯基取代烷基链可显着提高抑制效力和对FAAH的选择性。开发了两种高效的皮摩尔FAAH抑制剂(4c,IC 50 = 0.3±0.05 nM; 4d,IC 50 = 0.8±0.1 nM)。化合物4c以快速,选择性,非竞争性和不可逆的方式抑制FAAH。这项研究提供了几种高效和选择性的FAAH抑
-
Novel Uracil Derivatives: Newly Synthesized Centrally Acting Agents.作者:Masahiro IMAIZUMI、Fumitaka KANO、shinji SAKATADOI:10.1248/cpb.40.1808日期:——A series of 1-amino-5-substituted uracils and their 4-thio or 2,4-dithio substituted analogues were synthesized and assayed for anti-conflict activity in rats and anesthetic activity in mice. 1-Amino-5-halogenouracils 3b-e, 1-amino-4-thiouracil (9a), and 1-amino-5-halogeno-4-thiouracils 9c, d showed both anti-conflict and anesthetic activities. The most active compound was 1-amino-5-chloro-4-thiouracil
-
Synthesis of modified pyrimidine bases and positive impact of chemically reactive substituents on their in vitro antiproliferative activity作者:Steffi Noll、Marijeta Kralj、Lidija Šuman、Holger Stephan、Ivo PiantanidaDOI:10.1016/j.ejmech.2008.06.002日期:2009.3The antiproliferative activity screening on human tumor cell lines of a series of modified uracil and cytosine bases as well as some corresponding acyclonucleosides, and comparison of structure–activity relationship revealed the importance of chemical reactivity of the substituent attached to the C5-position of uracil for the activity of studied compounds. Namely, the results obtained for the most
-
The kinetics of the rearrangement of some isopyrimidines to pyrimidines studied by pulse radiolysis作者:Man Nien Schuchmann、Mohamed Al-Sheikhly、Clemens von Sonntag、Anthony Garner、George ScholesDOI:10.1039/p29840001777日期:——oxidation of the 6-yl radicals derived by ˙OH attack on pyrimidines and dihydropyrimidines. The Kinetics of the rearrangment of the isopyrimidines into the corresponding pyrimidines has been followed by pulse radiolysis. The rearrangement of isouracil into uracil is proton-catalysed (k 1.8 × 107 l mol–1 s–1). Around pH 7 a spontaneous reaction, k 3 000 s–1, is observed. On increasing the pH the isouracil异嘧啶是由asOH攻击嘧啶和二氢嘧啶而衍生的6-基团氧化而形成的。将异嘧啶重排成相应的嘧啶的动力学是通过脉冲辐射分解进行的。异尿嘧啶重排为尿嘧啶是质子催化的(k 1.8×10 7 l mol –1 s –1)。在pH值7左右,观察到自发反应k 3 000 s –1。随着pH值的增加,异尿嘧啶在N(3)处去质子化(p K a约为9.4)。所述isouracil阴离子的自发重排是相当慢(ķ ≤50秒1)。在pH> 10.5的OH - -催化反应集(ķ 4.9×10 5升摩尔1个小号-1),它涉及到一个第二去质子化,在C(5)。将5-羟基异尿嘧啶重排成异巴比妥酸也获得了相似的结果。阻断如3- methylisouracil的N(3)的位置,所述OH -在低得多的pH值诱发的重排在套(pH为9.5≤),即重排快(ķ 2.7×10 7升摩尔-1小号- 1),而不是其他两个系统中观察到的情况。
-
Measurement of Oxidative Damage at Pyrimidine Bases in γ-Irradiated DNA作者:Thierry Douki、Thierry Delatour、Frédérique Paganon、Jean CadetDOI:10.1021/tx960095b日期:1996.1.1Emphasis was placed in this work on the measurement of four oxidized pyrimidine bases, including 5-(hydroxymethyl)uracil (5-HMUra), 5-formyluracil (5-ForUra), 5-hydroxycytosine (5-OHCyt), and 5-hydroxyuracil (5-OHUra), in isolated DNA upon exposure to gamma radiation in aerated aqueous solution. For this purpose, both high performance liquid chromatography associated with electrochemical detection (HPLC-EC)氧化的核碱基代表了电离辐射在DNA中诱导的主要损伤类别之一。这项工作着重于测量四个氧化的嘧啶碱基,包括5-(羟甲基)尿嘧啶(5-HMUra),5-甲酰基尿嘧啶(5-ForUra),5-羟基胞嘧啶(5-OHCyt)和5-羟基尿嘧啶(5-OHUra),在曝气水溶液中暴露于伽玛射线后,分离的DNA中。为此,同时使用了与电化学检测相关的高效液相色谱(HPLC-EC)和与质谱联用的气相色谱(GC-MS)。仔细检查N-糖苷键的水解条件,以实现病变的定量释放。我们证明了60%的甲酸处理会导致所研究的四个病变的分解。另一方面,基于在吡啶中使用88%甲酸或70%氟化氢(HF / Pyr)进行的水解可以定量释放修饰的碱,但使用5-HMUra时除外。通过使用GC-MS分析进行剂量诱导的DNA中5-HMUra和5-ForUra形成的剂量过程研究表明,后一种病变的产生率比前一种病变高2.1倍。HF / Pyr和88
表征谱图
-
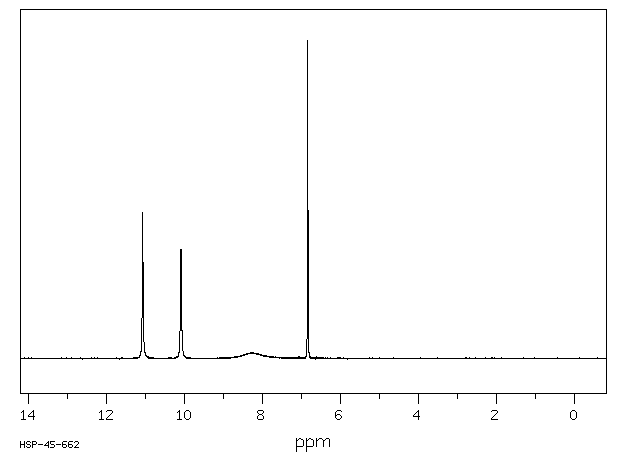
氢谱1HNMR
-
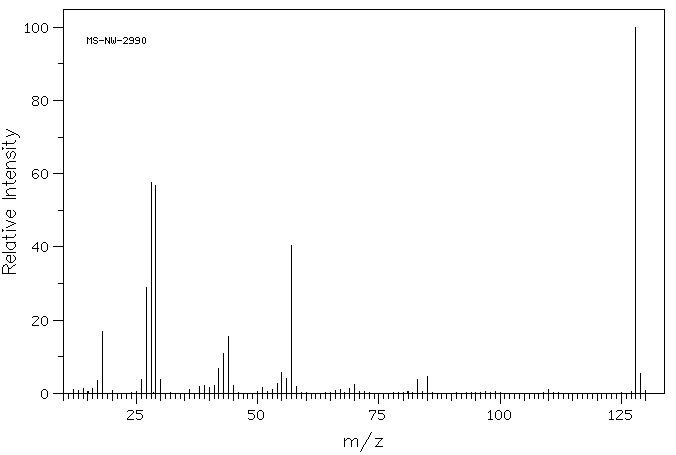
质谱MS
-
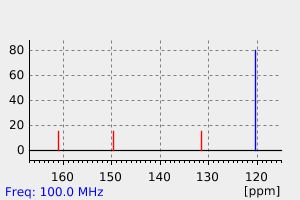
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-3-(2-(二氟甲基)吡啶-4-基)-7-氟-3-(3-(嘧啶-5-基)苯基)-3H-异吲哚-1-胺
(6-羟基嘧啶-4-基)乙酸
(4,5-二甲氧基-1,2,3,6-四氢哒嗪)
鲁匹替丁
马西替坦杂质7
马西替坦杂质4
马西替坦杂质
马西替坦原料药杂质D
马西替坦原料药杂质B
马西替坦
顺式-4-{[5-溴-2-(2,5-二甲基-1H-吡咯-1-基)-6-甲基嘧啶-4-基]氨基}环己醇
非沙比妥
非巴氨酯
非尼啶醇
青鲜素钾盐
雷特格韦钾盐
雷特格韦相关化合物E(USP)
雷特格韦杂质8
雷特格韦EP杂质H
雷特格韦-RT9
雷特格韦
阿西莫司杂质3
阿西莫司
阿脲四水合物
阿脲一水合物
阿维霉素
阿米美啶
阿米洛利
阿米妥钠
阿洛巴比妥
阿普瑞西他滨
阿普比妥
阿巴卡韦相关化合物B(USP)
阿卡明
阿伐那非杂质V
阿伐那非杂质1
阿伐那非杂质
阿伐那非中间体
阿伐那非
铂(2+)二氯化6-甲基-1,3-二{2-[(2-甲基丙基)硫烷基]乙基}嘧啶-2,4(1H,3H)-二酮(1:1)
钴1,2,3,6-四氢-2,6-二氧代嘧啶-4-羧酸酯(1:2)
钠5-烯丙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-乙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-(2-溴丙-2-烯基)-5-丁烷-2-基-4,6-二氧代-1H-嘧啶-2-醇
醌肟腙
酒石酸噻吩嘧啶
那可比妥
辛基2,6-二氧代-1,2,3,6-四氢-4-嘧啶羧酸酯
赛乐西帕杂质3
赛乐西帕KSM3