7-亚甲基十三烷 | 19780-80-4
中文名称
7-亚甲基十三烷
中文别名
2-己基-1-辛烯
英文名称
6-methylenetridecane
英文别名
7-methylenetridecane;2-hexyl-1-octene;2-hexyl-oct-1-ene;1,1-Di-(n-hexyl)-aethylen;1,1-Di-n-hexyl-ethylen;7-methylidenetridecane
CAS
19780-80-4
化学式
C14H28
mdl
MFCD00060974
分子量
196.376
InChiKey
QDOYJBSJTHIWKH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:95-97 °C(Press: 7 Torr)
-
密度:0.771±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):7.3
-
重原子数:14
-
可旋转键数:10
-
环数:0.0
-
sp3杂化的碳原子比例:0.857
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2901299090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 辛烯 oct-1-ene 111-66-0 C8H16 112.215
反应信息
-
作为反应物:描述:参考文献:名称:Hydroboration of Olefins with Catecholborane at Room Temperature in the Presence of N,N-Dimethylacetamide摘要:DOI:10.1021/jo960386p
-
作为产物:描述:三己基硼烷 、 1,2-dimethoxyethenyllithium 在 titanium(IV) isopropylate 、 sodium acetate 、 乙酸酐 、 四氯化钛 、 三氯乙酸 作用下, 生成 7-亚甲基十三烷参考文献:名称:从 1,2-二甲氧基乙烯基锂和三烷基硼烷生成对称 1,1-二烷基乙烯的便捷途径摘要:1,1-二烷基乙烯是由 1,2-二甲氧基乙烯基锂和有机硼烷通过用三氯乙酸处理,然后用乙酸钠-乙酸酐和 TiCl4/Ti(OPri)4 处理来制备的。DOI:10.1246/cl.1980.591
文献信息
-
Rh(II)-Catalyzed [2,3]-Sigmatropic Rearrangement of Sulfur Ylides Derived from Cyclopropenes and Sulfides作者:Hang Zhang、Bo Wang、Heng Yi、Yan Zhang、Jianbo WangDOI:10.1021/acs.orglett.5b01542日期:2015.7.2Rh2(OAc)4-catalyzed [2,3]-sigmatropic rearrangement of sulfur ylides is reported. A series of cyclopropenes were successfully employed for [2,3]-sigmatropic rearrangement by a reaction with either allylic or propargylic sulfides. Under the optimized conditions, the reaction afforded the products in moderate to excellent yields. In these transformations, the vinyl metal carbenes generated in situ from the cyclopropenes
-
Mild Ring‐Opening 1,3‐Hydroborations of Non‐Activated Cyclopropanes作者:Di Wang、Xiao‐Song Xue、Kendall N. Houk、Zhuangzhi ShiDOI:10.1002/anie.201811036日期:2018.12.17The Brown hydroboration reaction, first reported in 1957, is the addition of B−H across an olefin in an anti‐Markovnikov fashion. Here, we solved a long‐standing problem on mild 1,3‐hydroborations of non‐activated cyclopropanes. A three‐component system including cyclopropanes, boron halides, and hydrosilanes has been developed for borylative ring‐opening of cyclopropanes following the anti‐Markovnikov
-
B(C<sub>6</sub> F<sub>5</sub> )<sub>3</sub> -Catalyzed Ring Opening and Isomerization of Unactivated Cyclopropanes作者:Zi-Yu Zhang、Zhi-Yun Liu、Rui-Ting Guo、Yu-Quan Zhao、Xiang Li、Xiao-Chen WangDOI:10.1002/anie.201700864日期:2017.3.27Catalytic amounts of B(C6F5)3 promote the ring opening and subsequent isomerization of a series of unactivated cyclopropanes to afford terminal olefins in good yields when a hydrosilane and 2,6‐dibromopyridine are employed as additives.
-
Controllable, Sequential, and Stereoselective C–H Allylic Alkylation of Alkenes作者:Ling Qin、Mohammed Sharique、Uttam K. TambarDOI:10.1021/jacs.9b08801日期:2019.10.30new C-C bonds represents a powerful approach to generate complex molecules from simple starting materials. However, a general and controllable method for the sequential conversion of a methyl group into a fully substituted carbon center remains a challenge. We report a new method for the selective and sequential replacement of three C-H bonds at the allylic position of propylene and other simple terminal
-
The reaction of α-methoxyvinyllithium with trialkylboranes作者:Alan B. Levy、Steven J. Schwartz、Nancy Wilson、Bradley ChristieDOI:10.1016/s0022-328x(00)84870-6日期:1978.8Several alkenyltrialkylborate salts derived from the reaction of α-methoxyvinyllithium (MVL) with trialkylboranes have been prepared. At −80°C, the initial complex is stable as indicated by its iodination to give moderate yields of enol ethers. Warming the complex to room temperature leads via an alkyl group migration to a new alkenyldialkylmethoxyborate salt. Oxidation of this complex leads to methyl
表征谱图
-
氢谱1HNMR
-
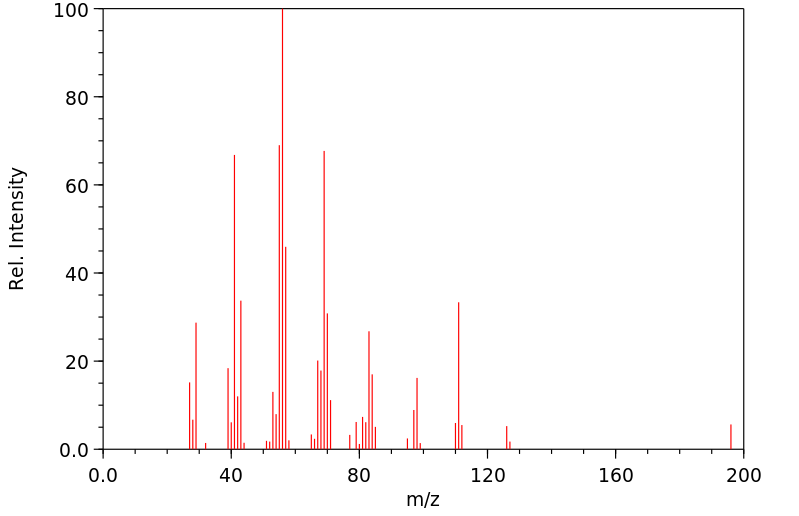
质谱MS
-
碳谱13CNMR
-
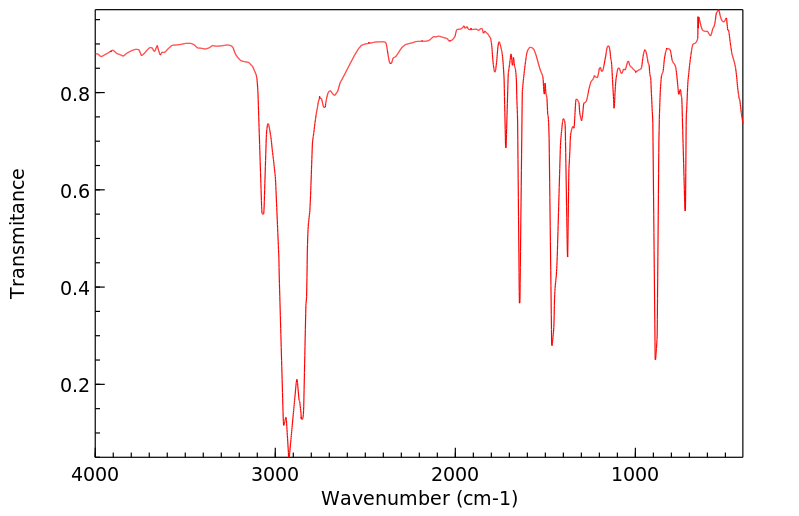
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-