L-酒石酸二丁酯 | 87-92-3
中文名称
L-酒石酸二丁酯
中文别名
L-(+)-酒石酸二丁酯
英文名称
(+)-(2R,3R)-dibutyl tartrate
英文别名
L(+) tartaric acid dibutyl ester;dibutyl-(l)-(+)-tartrate;(R,R)-dibutyl tartrate;di-n-butyl-L-tartrate;dibutyl L-tartrate;dibutyl-L-tartrate;Dibutyl tartrate;dibutyl (2R,3R)-2,3-dihydroxybutanedioate
CAS
87-92-3
化学式
C12H22O6
mdl
——
分子量
262.303
InChiKey
PCYQQSKDZQTOQG-NXEZZACHSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:20-22 °C
-
沸点:182 °C (11 mmHg)
-
密度:1.09
-
闪点:167 °C
-
溶解度:氯仿(微溶)、乙酸乙酯(微溶)
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:18
-
可旋转键数:11
-
环数:0.0
-
sp3杂化的碳原子比例:0.83
-
拓扑面积:93.1
-
氢给体数:2
-
氢受体数:6
安全信息
-
安全说明:S24/25
-
海关编码:2918130000
-
储存条件:密封保存,应存放在阴凉干燥处。
SDS
| Name: | Dibutyl L-Tartrate Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 87-92-3 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 87-92-3 | N-Dibutyl Tartrate | ca 100 | 201-784-9 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation. The toxicological properties of this material have not been fully investigated.
Skin:
May cause skin irritation. The toxicological properties of this material have not been fully investigated.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Use water spray to keep fire-exposed containers cool. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. Containers may explode when heated.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Cool containers with flooding quantities of water until well after fire is out. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Remove all sources of ignition. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 87-92-3: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Viscous liquid
Color: clear, colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: 167 deg C ( 332.60 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 1.0900g/cm3
Molecular Formula: C12H22O6
Molecular Weight: 262.29
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 87-92-3 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
N-Dibutyl Tartrate - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 87-92-3: No information available.
Canada
CAS# 87-92-3 is listed on Canada's NDSL List.
CAS# 87-92-3 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 87-92-3 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
概述
由于手性化合物尤其是手性药物的两个对映体具有不同的化学性质和生理活性,近年来对手性分离的关注度日益增加。L-酒石酸二丁酯不仅可用于手性化合物的拆分,还可作为硝酸纤维素、酯酸纤维素的增塑剂。特别地,在与磷酸三甲苯酯混合使用时,可以在硝酸纤维素中生成稳定的耐水涂膜;在酯酸纤维素中与苄醇混合使用时,同样可以形成类似的涂膜。
应急处理方法如果吸入L-酒石酸二丁酯,请立即将患者移至新鲜空气处;若皮肤接触到该物质,请脱去污染衣物,并用肥皂水和清水彻底清洗受污染的皮肤,如有不适,请及时就医;如眼睛接触,则需分开眼睑,使用流动清水或生理盐水冲洗,并立即寻求医疗帮助;如果误食,应立刻漱口并禁止催吐,随后尽快就医。
应用L-酒石酸二丁酯可用作硝酸纤维素和酯酸纤维素的增塑剂。尤其在与磷酸三甲苯酯混合使用时,在硝酸纤维素中可以生成稳定的耐水涂膜;而在酯酸纤维素中与苄醇结合,则同样能够形成类似的效果。此外,它还被用于制备提高复方丁公藤注射液安全性的药物组合物。
上下游信息
反应信息
-
作为反应物:参考文献:名称:立体化学方面-I:某些二醇的性质和反应摘要:分子内氢键的如通过羟基拉伸区域红外光谱,在某些邻二醇确定的范围内,环己烷,环戊烷,四氢吡喃和四氢呋喃和相关化合物提供了用于不同的构象的稳定性的证据。在某些化合物中,这些稳定性可能会受到取代基羟基与环氧之间氢键的影响。对于四氢吡喃二醇,通过[ M ] D值提供了其他证据。这些环状体系的邻位二醇与二醇拆分剂的反应速率及其在碱性硼酸盐缓冲液中的区带电泳迁移率受环氧的影响。DOI:10.1016/0040-4020(58)80056-3
-
作为产物:描述:dibenzyl (2R,3R)-2,3-dihydroxybutanedioate 、 正丁醇 以 various solvent(s) 为溶剂, 反应 24.0h, 以86.2%的产率得到L-酒石酸二丁酯参考文献:名称:A convenient method for enzymatic benzyl-alkyl transesterification under mild neutral conditions摘要:Lipases from Candida cylindracea and from Pseudomonas fluorescens efficiently catalyse the benzyl to alkyl transesterification in organic solvents under mild conditions in nearly quantitative yields.DOI:10.1016/s0040-4020(01)89450-3
-
作为试剂:参考文献:名称:Complexation of Vinylcyclopropanes with Zirconocene−1-butene Complex: Application to the Stereocontrolled Synthesis of Steroidal Side Chains摘要:Reactions of vinylcyclopropane derivatives with a zirconocene-1-butene complex (''Cp2Zr'') caused regioselective cleavage of the cyclopropyl bond to give eta(3) pi-allylic and/or eta(1) sigma-allylic complexes. The regioselectivity of the bond cleavage and the formation of a eta(3) pi-allylic or eta(1) sigma-allylic complex depend on the bulkiness of the substituents on the cyclopropyl group and the presence of leaving functionality. A catalytic use of ''Cp2Zr'' in the presence of excess Grignard reagent also caused a ring opening of the vinylcyclopropane derivatives with the same sense of regiochemical selectivity. The reaction of the thermally equilibrated ''Cp2Zr''-propenylcyclopropane complex with acetone indicated the possibility for the synthesis of the steroidal side chain in either natural or unnatural forms. The synthetic utility of the present ''Cp2Zr''-vinylcyclopropane chemistry was ascertained by the stereocontrolled preparation of the C-20, C-24 dimethylated steroidal side chain which is identical to the active metabolite of vitamin D-2.DOI:10.1021/jo962064r
文献信息
-
Imidazolium-based chiral ionic liquids: synthesis and application作者:Yumiko Suzuki、Junichiro Wakatsuki、Mariko Tsubaki、Masayuki SatoDOI:10.1016/j.tet.2013.09.017日期:2013.11centers on N-substituents is reported. [(2S,3S)-2,3-Dihydroxybutane-1,4-bis(3-butylimidazolium)]-[bis(trifluoromethanesulfonyl)amide]2 and [(4S,5S)-2-phenyl-1,3-dioxolane-4,5-bis(1-methylimidazolium)]-[bis(trifluoromethanesulfonyl)amide]2 induced enantioselectivity in the Michael addition of malonic esters to chalcones.
-
PHOSPHORYLATED POLYOLS, PYROPHOSPHATES, AND DERIVATIVES THEREOF HAVING BIOLOGICAL ACTIVITY申请人:NormOxys, Inc.公开号:US20140142052A1公开(公告)日:2014-05-22The present invention provides phosphorylated and pyrophosphate derivatives of polyols, and structural derivatives of these compounds, and provides pharmaceutical compositions comprising the same. The compounds and compositions disclosed herein have various biological activities, including for example, as allosteric effectors of hemoglobin and/or as kinase inhibitors. The present invention further provides methods for therapy in human or mammalian patients, and methods for synthesis of biologically active compounds and their intermediates.
-
[EN] PROCESS FOR PREPARATION OF ACITRETIN<br/>[FR] PROCÉDÉ DE PRÉPARATION D'ACITRÉTINE申请人:EMCURE PHARMACEUTICALS LTD公开号:WO2016042573A1公开(公告)日:2016-03-24The present invention provides a process for preparation of (2E,4E,6E,8E)-9-(4-methoxy-2,3,6- trimethyl)phenyl-3,7-dimethyl-nona-2,4,6,8}tetraenoate, an acitretin intermediate of formula (VI) with trans isomer ≥97%, comprising of reacting 3-formyl-crotonic acid butyl ester of formula (V), substantially free of impurities, with 5-(4-methoxy-2,3,6-trimethylphenyl)-3- methyl-penta-2,4-diene-l-triphenyl phosphonium bromide of formula (IV) and isolating resultant compound of formula (VI), treating the filtrate with iodine for isomerization of the undesired cis intermediate and finally obtaining acitretin (I), with desired trans isomer ≥97%.
-
Chiral induction in a biomimetic olefin cyclization作者:Hideyuki Takenaka、Tomohiro Sato、Mugio NishizawaDOI:10.1016/s0040-4039(00)99667-9日期:1989.1Chiral induction has been achieved during a biomimetic cyclization of a chiral perillene derivatives in maximum 76% diastereomeric excess. The absolute configuration of the predominant products are established by X-ray diffraction study and chemical transformations.
-
Asymmetric synthesis of (α-amino)phosphonic acid amphiphiles using chiral P–H spirophosphoranes作者:Christophe Déjugnat、Guita Etemad-Moghadam、Isabelle Rico-LattesDOI:10.1039/b304420c日期:——Chiral PâH spirophosphoranes reacted with long-chain prochiral aldimines and, after selective hydrolysis, afforded (α-amino)phosphonic acid amphiphiles in both enantiopure forms.
表征谱图
-
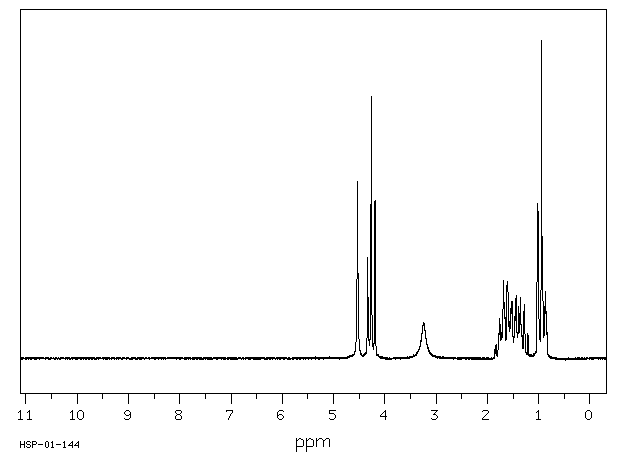
氢谱1HNMR
-
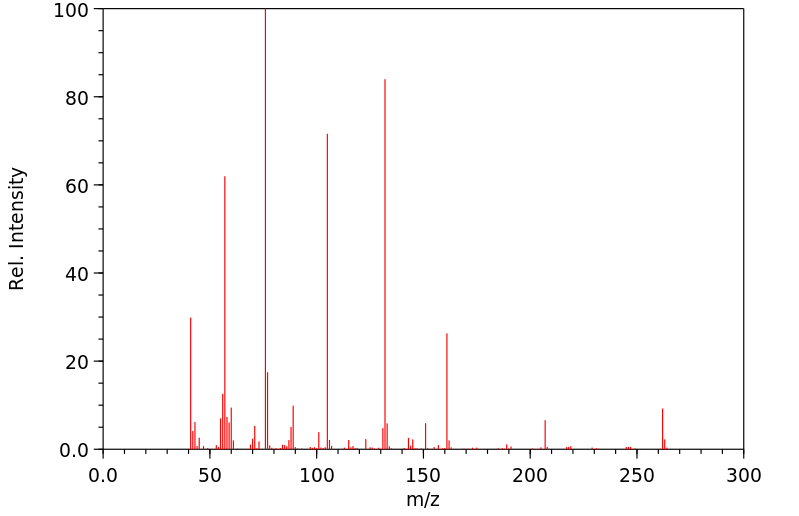
质谱MS
-
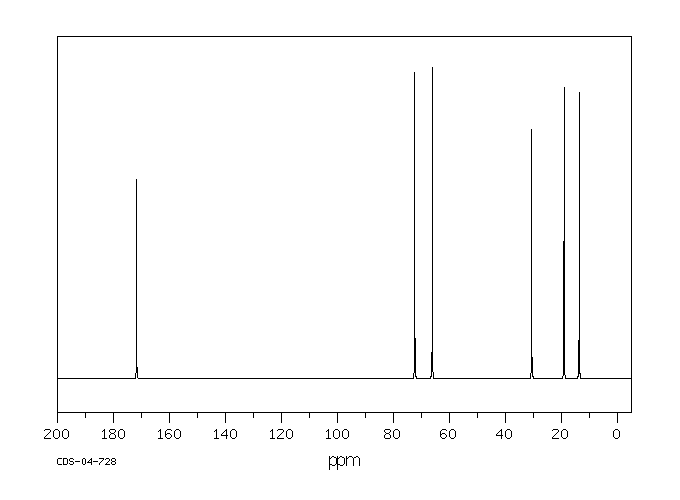
碳谱13CNMR
-
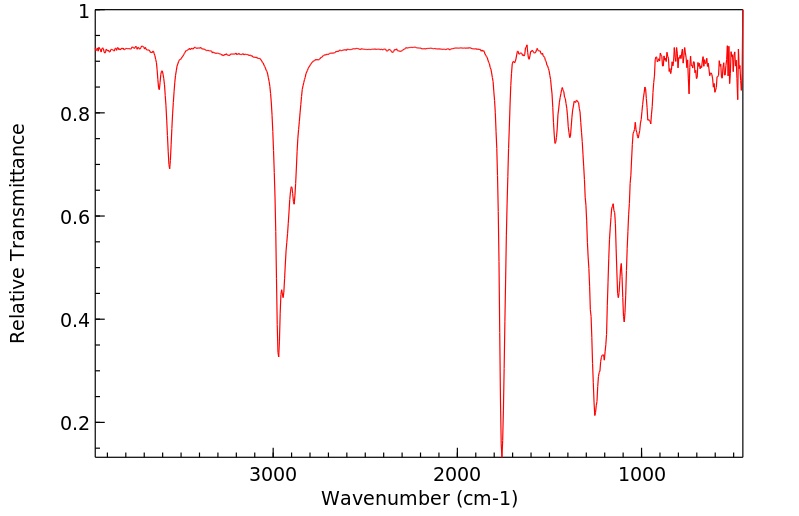
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-2-苯基-3-羟基丙酸
(2S,3R)-2,3-二羟基-3-(2-吡啶基)丙酸乙酯,N-氧化物
麦拉乳酸
阿拉伯碳酸氢二钾
铵;铈(+3)阳离子;(2R,3R)-2,3-二羟基丁烷二酸盐
钡二{8-[3-(2-羟基辛基)-2-环氧乙烷基]辛酸酯}
钠3-脱氧-D-阿拉伯糖-己酮酸酯
钠3-脱氧-D-木糖基-己酮酸酯
钠(3R,5R)-3,5-二羟基-7-[(1S,2S,6R,8S,8aR)-8-羟基-2,6-二甲基-1,2,6,7,8,8A-六氢-1-萘基]庚酸酯
钠(2S)-2-羟基(13C3)丙酸酯
酮酯
酒石酸锂单水合物
酒石酸铬
酒石酸铜(II)一水
酒石酸钾锑
酒石酸钾
酒石酸钠
酒石酸鐵(III)鉀
酒石酸辛酯钠盐
酒石酸羟吡啶
酒石酸氢钾
酒石酸异丙酯
酒石酸二磺基琥珀酰亚胺酯
酒石酸二琥珀酰亚胺酯
酒石酸二戊酯
酒石酸二仲丁酯
酒石酸二丙酯
辛酸,8-氯-6-羟基-,(6R)-
辛伐他汀钾盐
辛伐他汀钠盐
辛伐他汀酸
超支化BIS-MPA聚酯-64-羟基,4代
西托溴铵
表洛伐他汀羟基酸钠盐
葡萄糖酸镍
葡萄糖酸锶
葡萄糖酸锰
葡萄糖酸汞
葡萄糖酸亚铁
莫那可林J酸
苹果酸镁
苹果酸镁
苹果酸铵盐
苹果酸钙
苹果酸氢钠
苹果酸氢钠
苹果酸根
苹果酸二烯丙酯
苹果酸二乙基己酯
苹果酸乙酯(S)-2-羟基丁二酸1-乙酯(苹果酸杂质S)