苯磺酸正丁酯 | 80-44-4
中文名称
苯磺酸正丁酯
中文别名
苯磺酸丁酯
英文名称
n-butyl benzenesulfonate
英文别名
Benzolsulfonsaeure-n-butylester;Benzolsulfonsaeure-butylester;n-Butylbenzolsulfonat;Butyl benzenesulfonate
CAS
80-44-4
化学式
C10H14O3S
mdl
MFCD00025036
分子量
214.285
InChiKey
NIKBCKTWWPVAIC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:153 °C / 7mmHg
-
密度:1.15
-
溶解度:可溶于氯仿、甲醇
-
保留指数:1682
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:14
-
可旋转键数:5
-
环数:1.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:51.8
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险性防范说明:P261,P264,P271,P280,P302+P352,P304+P340+P312,P305+P351+P338,P332+P313,P337+P313,P362,P403+P233,P405,P501
-
危险性描述:H315,H319,H335
-
储存条件:存放于惰性气体中,并避免接触湿气(否则可能分解)。
SDS
Section I.Chemical Product and Company Identification
Chemical Name Benzenesulfonic Acid n-Butyl Ester
Portland OR
Synonym n-Butyl Benzenesulfonate
Chemical Formula C6H5SO3(CH2)3CH3
CAS Number 80-44-4
Section II. Composition and Information on Ingredients
Chemical Name CAS Number Percent (%) TLV/PEL Toxicology Data
Benzenesulfonic Acid n-Butyl Ester 80-44-4 ---------- Not available. Not available.
Section III. Hazards Identification
Acute Health Effects No specific information is available in our data base regarding the toxic effects of this material for humans. However,
exposure to any chemical should be kept to a minimum. Skin and eye contact may result in irritation. May be harmful if
inhaled or ingested. Always follow safe industrial hygiene practices and wear proper protective equipment when handling
this compound.
CARCINOGENIC EFFECTS : Not available.
Chronic Health Effects
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
There is no known effect from chronic exposure to this product. Repeated or prolonged exposure to this compound is
not known to aggravate existing medical conditions.
Section IV. First Aid Measures
Eye Contact Check for and remove any contact lenses. IMMEDIATELY flush eyes with runing water for at least 15 minutes. keeping
eyelids open. COLD water may be used. DO NOT use an eye oitment. Flush eyes with running water for a minimum of
15 minutes, occasionally lifting the upper eyelids. Seek medical attention. Treat symptomatically and supportively.
Skin Contact After contact with skin, wash immediately with plenty of water. Gently and thorough wash the contaminated skin with
running water and non-abrasive soap. Be particularly careful to clean folds, crevices, creases and groin. COLD water
may be used. Cover the irritated skin with an emollient. Seek medical attention. Treat symptomatically and supportively.
Wash any contaminated clothing before reusing.
Inhalation If the victim is not breathing, perform artificial respiration. Loosen tight clothing such as a collar, tie, belt or waistband. If
breathing is difficult, oxygen can be administered. Seek medical attention. Treat symptomatically and supportively.
Ingestion INDUCE VOMITING by sticking finger in throat. Lower the head so that the vomit will not reenter the mouth and throat.
Loosen tight clothing such as a collar, tie, belt, or waistband. If the victim is not breathing, administer artificial respiration.
Examine the lips and mouth to ascertain whether the tissues are damaged, a possible indication that the toxic material
was ingested; the absence of such signs, however, is not conclusive. Seek immediate medical attention and, if possible,
show the chemical label. Treat symptomatically and supportively.
Section V. Fire and Explosion Data
Auto-Ignition Not available.
Flammability May be combustible at high temperature.
Flash Points Flammable Limits Not available.
Not available.
Combustion Products These products are toxic carbon oxides (CO, CO 2), sulfur oxides (SO2, SO3...).
Fire Hazards
No specific information is available regarding the flammability of this compound in the presence of various materials.
Explosion Hazards Risks of explosion of the product in presence of mechanical impact: Not available.
Risks of explosion of the product in presence of static discharge: Not available.
No additional information is available regarding the risks of explosion.
Fire Fighting Media
SMALL FIRE: Use DRY chemicals, CO 2, water spray or foam.
and Instructions LARGE FIRE: Use water spray, fog or foam. DO NOT use water jet.
Continued on Next Page
Benzenesulfonic Acid n-Butyl Ester
Section VI. Accidental Release Measures
Spill Cleanup In case of a spill and/or a leak, always shut off any sources of ignition, ventilate the area, and exercise caution. Absorb
Instructions with an inert material and put the spilled material in an appropriate waste disposal. Finish cleaning the spill by rinsing any
contaminated surfaces with copious amounts of water. Consult federal, state, and/or local authorities for assistance on
disposal.
Section VII. Handling and Storage
Handling and Storage Keep away from heat and sources of ignition. Mechanical exhaust required. When not in use, tightly seal the container
and store in a dry, cool place. Avoid excessive heat and light. Do not breathe gas, fumes, vapor or spray.
Information
Always store away from incompatible compounds such as oxidizing agents.
Section VIII. Exposure Controls/Personal Protection
Engineering Controls Provide exhaust ventilation or other engineering controls to keep the airborne concentrations of vapors below their
respective threshold limit value. Ensure that eyewash station and safety shower is proximal to the work-station location.
Personal Protection Splash goggles. Lab coat. Vapor respirator. Boots. Gloves. Be sure to use a MSHA/NIOSH approved respirator or
equivalent. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling this product.
Exposure Limits Not available.
Section IX. Physical and Chemical Properties
Solubility
Physical state @ 20°C Liquid. Not available.
1.16
Specific Gravity
Molecular Weight 214.28 Partition Coefficient Not available.
Boiling Point Not available. Vapor Pressure Not available.
Melting Point Not available. Vapor Density Not available.
Not available. Volatility Not available.
Refractive Index
Critical Temperature Not available. Odor Not available.
Viscosity Not available. Taste Not available.
Section X. Stability and Reactivity Data
Stability
This material is stable if stored under proper conditions. (See Section VII for instructions)
Conditions of Instability Avoid excessive heat and light.
Incompatibilities
Reactive with oxidizing agents.
Section XI. Toxicological Information
RTECS Number Not available.
Eye contact. Ingestion. Inhalation.
Routes of Exposure
Toxicity Data Not available.
Chronic Toxic Effects CARCINOGENIC EFFECTS : Not available.
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
There is no known effect from chronic exposure to this product. Repeated or prolonged exposure to this compound is not
known to aggravate existing medical conditions.
Acute Toxic Effects No specific information is available in our data base regarding the toxic effects of this material for humans. However,
exposure to any chemical should be kept to a minimum. Skin and eye contact may result in irritation. May be harmful if
inhaled or ingested. Always follow safe industrial hygiene practices and wear proper protective equipment when handling
this compound.
Continued on Next Page
Benzenesulfonic Acid n-Butyl Ester
Section XII. Ecological Information
Ecotoxicity Not available.
Environmental Fate Not available.
Section XIII. Disposal Considerations
Recycle to process, if possible. Consult your local or regional authorities. You may be able to dissolve or mix material with
Waste Disposal
a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system. Observe all
federal, state, and local regulations when disposing of the substance.
Section XIV. Transport Information
DOT Classification Not a DOT controlled material (United States).
PIN Number Not applicable.
Proper Shipping Name
Not applicable.
Packing Group (PG) Not applicable.
DOT Pictograms
Section XV. Other Regulatory Information and Pictograms
TSCA Chemical Inventory This product is NOT on the EPA Toxic Substances Control Act (TSCA) inventory. The following notices are required by 40
CFR 720.36 (C) for those products not on the inventory list:
(EPA)
(i) These products are supplied solely for use in research and development by or under the supervision of a technically
qualified individual as defined in 40 CFR 720.0 et sec.
(ii) The health risks of these products have not been fully determined. Any information that is or becomes available will be
supplied on an MSDS sheet.
WHMIS Classification Not available.
(Canada)
EINECS Number (EEC) Not available.
EEC Risk Statements Not available.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:参考文献:名称:Truchet; Graves, Bulletin de la Societe Chimique de France, 1932, vol. <4> 51, p. 688摘要:DOI:
-
作为产物:描述:α,α-diphenyl-o-(N-methyl-N-phenylsulfamyl)benzyl alcohol 在 2,2,6,6-tetramethylpiperidinyl-lithium 、 水 作用下, 以 四氢呋喃 为溶剂, 反应 5.0h, 生成 苯磺酸正丁酯参考文献:名称:Intermediacy of o-Sulfonylium Arenide Ylides in the Reactions of Arenesulfonyl Derivatives with Strong Bases: Insight into the Puzzling Rearrangement of N-Arylarenesulfonamides into 2-Aminodiaryl Sulfones1摘要:Benzenesulfonyl derivatives, PhSO(2)E, such as benzenesulfonyl fluoride, phenyl benzenesulfonate, and N-methyl-N-phenylbenzenesulfonamide, have been found to undergo ortho-lithiation with lithium 2,2,6,6-tetramethylpiperidide (LTMP) in THF at -78 degrees C. The resulting lithio derivatives undergo 1,3-elimination of LiE to form the transient o-sulfonylium benzenide. The latter ylide, which may be stabilized by its carbenoid character and its complexation with LiE, can be trapped by benzophenone acting as a 1,3-dipolarophile to form the adduct, 3,3-diphenyl-1,2-benzoxathiole 1,1-dioxide. The alternative formation of the latter cyclic sulfonate by reaction of the initially formed o-lithio derivative with benzophenone and by subsequent cyclization was shown not to occur. If not trapped with benzophenone, such an ylide decomposes partly into SO2 and benzyne and then undergoes self-condensation to yield poly(phenylene-o,o'-biphenylene sulfones), o-Sulfonylium benzenide also can be captured by lithium alkoxides, either present adventitously or intentionally added, to generate the corresponding alkyl benzenesulfonate. However, such alkoxides, unaided by LTMP, are themselves unable to extract an ortho-proton from benzenesulfonyl fluoride or from N-methyl-N-phenylbenzenesulfonamide to yield the corresponding o-lithio derivative. With benzenesulfonyl fluoride the lithium alkoxide can also form the sulfonate ester by direct substitution at the sulfonyl group. The Closson-Hellwinkel rearrangement of N-methyl-N-phenylbenzenesulfonamide into o-(methylamino)diphenyl sulfone by RLi or LTMP is reinterpreted as proceeding by way of the 2-lithio- or 2,6-dilithiobenzenesulfonyl derivative which eliminates lithium N-methylanilide to form the o-sulfonylium benzenide or its 6-lithio derivative. Attack of the latter ylide, acting as an electrophile, upon the ortho-position of the presumably complexed LiNMePh then consummates the rearrangement.DOI:10.1021/jo950727j
文献信息
-
Sequential C–S and S–N Coupling Approach to Sulfonamides作者:Kai Chen、Wei Chen、Bing Han、Wanzhi Chen、Miaochang Liu、Huayue WuDOI:10.1021/acs.orglett.0c00183日期:2020.3.6A one-pot three-component reaction involving nitroarenes, (hetero)arylboronic acids, and potassium pyrosulfite leading to sulfonamides was described. A broad range of sulfonamides bearing different reactive functional groups were obtained in good to excellent yields through sequential C-S and S-N coupling that does not require metal catalysts.
-
METHODS OF SYNTHESIZING A POLYNUCLEOTIDE ARRAY USING PHOTOACTIVATED AGENTS申请人:Vibrant Holdings, LLC公开号:US20210380629A1公开(公告)日:2021-12-09Described herein are methods for the synthesis of DNA polynucleotides and polynucleotides, as well as methods for their deprotection and methods for the use of said compounds and compositions comprising said compounds. In particular, such compounds and compositions comprising them are used in methods for light-directed synthesis of DNA microarrays.本文描述了合成DNA多核苷酸和多核苷酸的方法,以及它们的去保护方法、使用这些化合物的方法和包含这些化合物的组合物的方法。特别是,这些化合物和包含它们的组合物用于DNA微阵列的光导向合成方法。
-
[EN] CARBOSTYRIL COMPOUND<br/>[FR] DÉRIVÉ DE CARBOSTYRILE申请人:OTSUKA PHARMA CO LTD公开号:WO2006035954A1公开(公告)日:2006-04-06The present invention provides a carbostyril compound represented by General Formula (1) or a salt thereof, wherein A is a direct bond, a lower alkylene group, or a lower alkylidene group; X is an oxygen atom or a sulfur atom; R4 and R5 each represent a hydrogen atom; the bond between the 3 and 4 positions of the carbostyril skeleton is a single bond or a double bond; R1 is a hydrogen atom, etc; R2 is a hydrogen atom, etc; and R3 is a hydrogen atom, etc. The carbostyril compound or salt thereof of the present invention induces the production of TFF, and thus is usable for the treatment and/or prevention of disorders such as alimentary tract diseases, oral diseases, upper respiratory tract diseases, respiratory tract diseases, eye diseases, cancers, and wounds.
-
MANUFACTURING METHOD FOR POLYCARBONATE申请人:——公开号:US20020037838A1公开(公告)日:2002-03-28A method for manufacturing polycarbonate by melt-polycondensing bisphenol and carbonic acid diester uses as catalyst an alkali metal compound and/or alkaline earth metal compound (a). The catalyst is added to the bisphenol prior to the melt polycondensation, in an effective amount, i.e., the amount of alkali metal compound and/or alkaline earth metal compound (a) that acts effectively as a catalyst, is contained in said bisphenol, and is controlled to have the same catalytic activity as 1×10 −8 to 1×10 −6 mole of bisphenol disodium salt per mole of pure bisphenol A. The method conducts the reaction efficiently from the initial stage in a stable manner to obtain polycarbonate with good color, good heat stability and color stability during molding and the like.
-
5-ALA ester formulations and use thereof申请人:UNIVERSITE DE GENEVE公开号:EP2394642A1公开(公告)日:2011-12-14The present invention is directed to particles comprising 5-ALA esters salts, formulation thereof, related methods of preparation and methods of use thereof. In particular, the invention relates to particles comprising 5-ALA esters salts, formulation thereof useful in the treatment of a cancer and/or the diagnosis of a cancer cell such as in photodynamic therapy or photodynamic diagnosis.
表征谱图
-
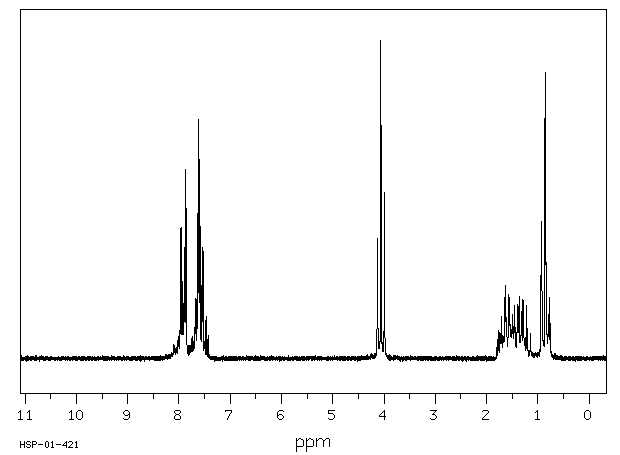
氢谱1HNMR
-
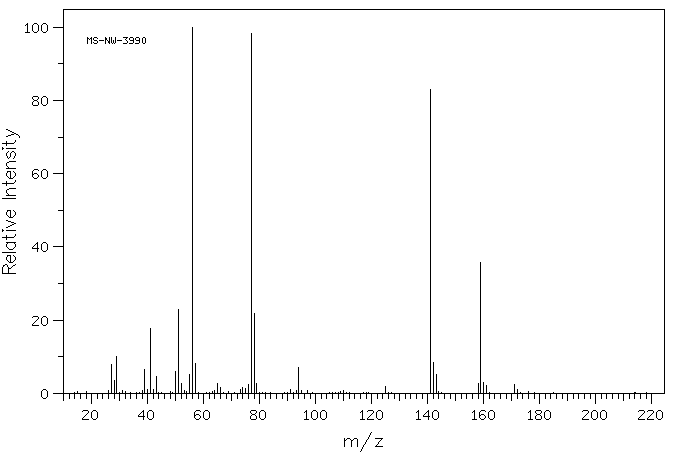
质谱MS
-
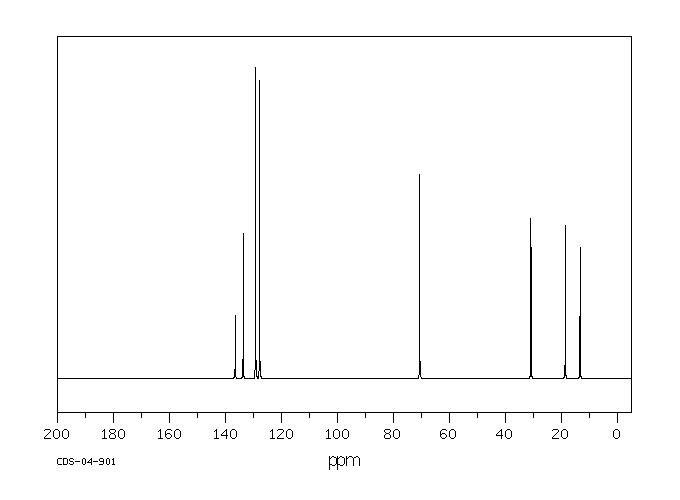
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫