N-乙基正丙胺 | 20193-20-8
物质功能分类
中文名称
N-乙基正丙胺
中文别名
N-乙基丙胺;α-乙基丙胺;二乙基甲胺;N-乙基-n-丙胺
英文名称
ethylpropylamine
英文别名
N-ethylpropylamine;N-ethyl-n-propylamine;N-ethylpropan-1-amine
CAS
20193-20-8
化学式
C5H13N
mdl
MFCD00015198
分子量
87.1649
InChiKey
XCVNDBIXFPGMIW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-75°C (estimate)
-
沸点:80-85°C
-
密度:0,72 g/cm3
-
闪点:80-85°C
-
溶解度:可溶于氯仿、乙酸乙酯
-
保留指数:652
-
稳定性/保质期:
如果遵照规格使用和储存,则不会分解。避免接触氧化物和酸。
计算性质
-
辛醇/水分配系数(LogP):1.1
-
重原子数:6
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:12
-
氢给体数:1
-
氢受体数:1
安全信息
-
TSCA:Yes
-
危险等级:3
-
危险品标志:F,T,C
-
安全说明:S20,S26,S36/37/39,S45
-
危险类别码:R34
-
危险品运输编号:2735
-
海关编码:2921199090
-
危险类别:3
-
包装等级:II
-
储存条件:保持贮藏器密封,并将其放入一个紧密封装的容器中。应储存在阴凉、干燥的地方。
SDS
N-Ethylpropylamine Revision number: 6
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: N-Ethylpropylamine
Revision number: 6
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS
Category 2
Flammable liquids
HEALTH HAZARDS
Category 1B
Skin corrosion/irritation
Serious eye damage/eye irritation Category 1
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Danger
Hazard statements Highly flammable liquid and vapour
Causes severe skin burns and eye damage
Precautionary statements:
[Prevention] Keep away from heat/sparks/open flames/hot surfaces. - No smoking.
Keep container tightly closed.
Use explosion-proof electrical/ventilating/lighting equipment. Take precautionary
measures against ignition by the static discharge and the spark.
Do not breathe dust/fume/gas/mist/vapours/spray.
Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
[Response]
breathing.
IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
Wash contaminated clothing before reuse.
Immediately call a POISON CENTER or doctor/physician.
[Storage] Store in a well-ventilated place. Keep cool.
Store locked up.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
N-Ethylpropylamine
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: N-Ethylpropylamine
Percent: >97.0%(GC)
CAS Number: 20193-20-8
Chemical Formula: C5H13N
Section 4. FIRST AID MEASURES
Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Inhalation:
Immediately call a POISON CENTER or doctor/physician.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. Immediately call a POISON CENTER or doctor/physician.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing.Immediately call a POISON CENTER or
doctor/physician.
Ingestion: Immediately call a POISON CENTER or doctor/physician. Rinse mouth. Do NOT
induce vomiting.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, carbon dioxide.
media:
Unsuitable extinguishing Water (It may scatter and spread fire.)
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Keep containers cool by
spraying with water. Eliminate all ignition sources if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use extra personal protective equipment (self-contained breathing apparatus). Keep
protective equipment and people away from and upwind of spill/leak. Ensure adequate ventilation. Entry to non-
emergency procedures: involved personnel should be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in dry sand or inert absorbent before recovering it into an
containment and cleaning airtight container. In case of large amount of spillage, contain a spill by bunding.
up: Adhered or collected material should be promptly disposed of, in accordance with
appropriate laws and regulations.
Prevention of secondary Remove all sources of ignition. Fire-extinguishing devices should be prepared in
hazards: case of a fire. Use spark-proof tools and explosion-proof equipment.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Keep away from heat/sparks/open flame/hot
surfaces. -No smoking. Take measures to prevent the build up of electrostatic
charge. Use explosion-proof equipment. Wash hands and face thoroughly after
handling.
Use a closed system if possible. Use a ventilation, local exhaust if vapour or aerosol
will be generated.
Avoid contact with skin, eyes and clothing.
Advice on safe handling:
N-Ethylpropylamine
Section 7. HANDLING AND STORAGE
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool, dark and well-ventilated place.
Store under inert gas.
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Air-sensitive
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust. Also install safety shower and eye bath.
Engineering controls:
Personal protective equipment
Respiratory protection: Half or full facepiece respirator, self-contained breathing apparatus(SCBA), supplied
air respirator, etc. Use respirators approved under appropriate government standards
and follow local and national regulations.
Hand protection: Impervious gloves.
Safety goggles. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Liquid
Physical state (20°C):
Form: Clear
Colour: Colorless - Very pale yellow
No data available
Odour:
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: 79°C
4°C
Flash point:
Flammability or explosive
limits:
Lower: No data available
No data available
Upper:
Relative density: 0.72
Solubility(ies):
[Water] No data available
No data available
[Other solvents]
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Conditions to avoid: Spark, Open flame, Static discharge
Incompatible materials: Oxidizing agents, Strong acids
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
N-Ethylpropylamine
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
No data available
Log Pow:
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system but exert extra care in igniting as this material is
highly flammable. Observe all federal, state and local regulations when disposing of the substance
Section 14. TRANSPORT INFORMATION
3: Flammable liquid.
Hazards Class:
Subsidiary risk: 8: Corrosive.
UN-No: 2733
Proper shipping name: Amines, flammable, corrosive, n.o.s.
Packing group: II
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: N-Ethylpropylamine
Revision number: 6
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS
Category 2
Flammable liquids
HEALTH HAZARDS
Category 1B
Skin corrosion/irritation
Serious eye damage/eye irritation Category 1
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Danger
Hazard statements Highly flammable liquid and vapour
Causes severe skin burns and eye damage
Precautionary statements:
[Prevention] Keep away from heat/sparks/open flames/hot surfaces. - No smoking.
Keep container tightly closed.
Use explosion-proof electrical/ventilating/lighting equipment. Take precautionary
measures against ignition by the static discharge and the spark.
Do not breathe dust/fume/gas/mist/vapours/spray.
Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
[Response]
breathing.
IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
Wash contaminated clothing before reuse.
Immediately call a POISON CENTER or doctor/physician.
[Storage] Store in a well-ventilated place. Keep cool.
Store locked up.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
N-Ethylpropylamine
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: N-Ethylpropylamine
Percent: >97.0%(GC)
CAS Number: 20193-20-8
Chemical Formula: C5H13N
Section 4. FIRST AID MEASURES
Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Inhalation:
Immediately call a POISON CENTER or doctor/physician.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. Immediately call a POISON CENTER or doctor/physician.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing.Immediately call a POISON CENTER or
doctor/physician.
Ingestion: Immediately call a POISON CENTER or doctor/physician. Rinse mouth. Do NOT
induce vomiting.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, carbon dioxide.
media:
Unsuitable extinguishing Water (It may scatter and spread fire.)
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Keep containers cool by
spraying with water. Eliminate all ignition sources if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use extra personal protective equipment (self-contained breathing apparatus). Keep
protective equipment and people away from and upwind of spill/leak. Ensure adequate ventilation. Entry to non-
emergency procedures: involved personnel should be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in dry sand or inert absorbent before recovering it into an
containment and cleaning airtight container. In case of large amount of spillage, contain a spill by bunding.
up: Adhered or collected material should be promptly disposed of, in accordance with
appropriate laws and regulations.
Prevention of secondary Remove all sources of ignition. Fire-extinguishing devices should be prepared in
hazards: case of a fire. Use spark-proof tools and explosion-proof equipment.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Keep away from heat/sparks/open flame/hot
surfaces. -No smoking. Take measures to prevent the build up of electrostatic
charge. Use explosion-proof equipment. Wash hands and face thoroughly after
handling.
Use a closed system if possible. Use a ventilation, local exhaust if vapour or aerosol
will be generated.
Avoid contact with skin, eyes and clothing.
Advice on safe handling:
N-Ethylpropylamine
Section 7. HANDLING AND STORAGE
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool, dark and well-ventilated place.
Store under inert gas.
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Air-sensitive
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust. Also install safety shower and eye bath.
Engineering controls:
Personal protective equipment
Respiratory protection: Half or full facepiece respirator, self-contained breathing apparatus(SCBA), supplied
air respirator, etc. Use respirators approved under appropriate government standards
and follow local and national regulations.
Hand protection: Impervious gloves.
Safety goggles. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Liquid
Physical state (20°C):
Form: Clear
Colour: Colorless - Very pale yellow
No data available
Odour:
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: 79°C
4°C
Flash point:
Flammability or explosive
limits:
Lower: No data available
No data available
Upper:
Relative density: 0.72
Solubility(ies):
[Water] No data available
No data available
[Other solvents]
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Conditions to avoid: Spark, Open flame, Static discharge
Incompatible materials: Oxidizing agents, Strong acids
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
N-Ethylpropylamine
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
No data available
Log Pow:
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system but exert extra care in igniting as this material is
highly flammable. Observe all federal, state and local regulations when disposing of the substance
Section 14. TRANSPORT INFORMATION
3: Flammable liquid.
Hazards Class:
Subsidiary risk: 8: Corrosive.
UN-No: 2733
Proper shipping name: Amines, flammable, corrosive, n.o.s.
Packing group: II
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— Ethyl-n-propyl-hydroxylamin 40548-47-8 C5H13NO 103.164 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N-乙基-N-甲基-1-丙胺 N-methyl-N-ethylpropylamine 4458-32-6 C6H15N 101.192 3-(乙基氨基)-1-丙醇 3-ethylamino-propan-1-ol 42055-16-3 C5H13NO 103.164 —— N-ethyl-3-chloropropylamine —— C5H12ClN 121.61
反应信息
-
作为反应物:参考文献:名称:巴拉塞替的制备方法摘要:本发明公开了巴拉塞替的制备方法,所述巴拉塞替化学名称为2‑{5‑[(7‑{3‑[乙基(2‑羟基乙基)氨基]丙氧基}喹唑啉‑4‑基)氨基]‑1H‑吡唑‑3‑基}‑N‑(3‑氟苯基)乙酰胺,其化学式为C26H30FN7O3;本发明制备工艺过程简洁,原料易得,经济环保,有利于实现工业化,可促进巴拉塞替原料药的经济技术发展,降低了生成成本,适于大批量生产。公开号:CN107641115A
-
作为产物:参考文献:名称:在温和条件下将酰胺催化加氢为胺摘要:在(不是很大)压力下:已开发出一种使用双金属Pd-Re催化剂将叔酰胺和仲酰胺氢化成胺的通用方法,具有优异的选择性。反应在低压和较低温度下进行。该方法为有机化学家提供了一种简单可靠的胺合成工具。DOI:10.1002/anie.201207803
-
作为试剂:描述:1-甲基-1H-咪唑-5-甲酸 、 8H-茚并[1,2-d]噻唑-2-胺 、 三氟乙酸 在 N-乙基正丙胺 、 (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate 作用下, 以 二氯甲烷 、 水 、 乙腈 为溶剂, 反应 96.0h, 生成 3-methyl-3H-imidazole-4-carboxylic acid (8H-indeno[1,2-d]thiazol-2-yl)-amide trifluoroacetate参考文献:名称:Substituted N-acyl-2-aminothiazoles摘要:提供了该公式化合物或其药用可接受的盐,可用于治疗糖尿病、糖尿病视网膜病变、哮喘和腹泻。公开号:US20060030589A1
文献信息
-
Syntheses and Neuraminidase Inhibitory Activity of Multisubstituted Cyclopentane Amide Derivatives作者:Pooran Chand、Y. Sudhakar Babu、Shanta Bantia、Scott Rowland、Ali Dehghani、Pravin L. Kotian、Tracy L. Hutchison、Shoukath Ali、Wayne Brouillette、Yahya El-Kattan、Tsu-Hsing LinDOI:10.1021/jm0303406日期:2004.4.1position 3, were tested for their ability to inhibit A and B forms of influenza neuraminidase. The 1-ethylpropylamide, diethylamide, dipropylamide, and 4-morpholinylamide showed very good inhibitory activity (IC(50) = 0.015-0.080 microM) vs the neuraminidase A form, but modest activity (IC(50) = 3.0-9.2 microM) vs the neuraminidase B form. Since the parent amides bear two chiral centers (C-1 and C-1'), three在旨在鉴定有效和安全的流感神经氨酸酶抑制剂的进一步研究中,我们合成了一系列多取代的环戊烷酰胺衍生物。所制备的酰胺是伯胺的14个实例的N-取代烷基或芳烷基,仲胺的13个实例的N,N-二取代的烷基,芳烷基或取代烷基,以及脂环族或取代的脂环族仲胺的12个实例。这些化合物具有两个手性中心,分别位于环的位置1和位置3的侧链的位置1',并具有抑制甲型和乙型流感神经氨酸酶的能力。与神经氨酸酶A形式相比,1-乙基丙基酰胺,二乙基酰胺,二丙基酰胺和4-吗啉基酰胺显示出非常好的抑制活性(IC(50)= 0.015-0.080 microM),但相对于神经氨酸酶B形式,活性适中(IC(50)= 3.0-9.2 microM)。由于母体酰胺带有两个手性中心(C-1和C-1'),因此以较高的非对映异构体纯度测试了三种较好的抑制剂。与1-(乙基)丙基酰胺,二乙基酰胺和二丙基酰胺的活性形式相对应的非对映异构体(在C-1
-
A Roadmap to Uranium Ionic Liquids: Anti‐Crystal Engineering作者:Damla Yaprak、Eike T. Spielberg、Tobias Bäcker、Mark Richter、Bert Mallick、Axel Klein、Anja‐Verena MudringDOI:10.1002/chem.201303333日期:2014.5.19relationships regarding the ionic liquid formation capability. Compounds with the least symmetric N,N‐dialkyldithiocarbamato ligand and hence the least symmetric anions, tris(N‐methyl‐N‐propyldithiocarbamato)uranylate, tris(N‐ethyl‐N‐propyldithiocarbamato)uranylate, and tris(N‐methyl‐N‐butyldithiocarbamato)uranylate, lead to the formation of (room‐temperature) ionic liquids, which confirms that low‐symmetry在寻找基于铀的离子液体时,三(N,N-二烷基二硫代氨基甲酸酯)铀酰已合成为1-丁基-3-甲基咪唑鎓(C 4 mim)阳离子的盐。作为与UO 2 2+单元结合的二硫代氨基甲酸酯配体,四亚甲基,五亚甲基,六亚甲基和六亚甲基二硫代氨基甲酸酯,N,N-二乙基二硫代氨基甲酸酯,N-甲基-N-丙基二硫代氨基甲酸酯,N-乙基-N-丙基二硫代氨基甲酸酯和N-甲基‐ N已研究了丁基二硫代氨基甲酸酯。X射线单晶衍射可以对所有化合物进行明确的结构表征,但N-甲基-N-丁基二硫代氨基甲酸酯仅作为玻璃态材料获得。此外,在计算方法的支持下,使用粉末X射线衍射以及振动和UV / Vis光谱对产品进行了表征。差示扫描量热法用于研究取决于N,N-二烷基二硫代氨基甲酸酯配体的相变行为,旨在建立有关离子液体形成能力的结构-性质关系。N,N最不对称的化合物-二烷基二硫代氨基甲酸酯配体,因此最不对称的阴离子是三(N-甲基-N-丙基
-
[EN] 5-(1 H-BENZO[D]IMIDAZO-2-YL)-PYRIDIN-2-AMINE AND 5-(3H-IMIDAZO[4,5-B]PYRIDIN-6-YL)-PYRIDIN-2-AMINE DERIVATIVES AS C-MYC AND P300/CBP HISTONE ACETYLTRANSFERASE INHIBITORS FOR TREATING CANCER<br/>[FR] DÉRIVÉS DE 5-(1 H-BENZO[D]IMIDAZO-2-YL)-PYRIDIN-2-AMINE ET DE 5-(3H-IMIDAZO[4,5-B]PYRIDIN-6-YL)-PYRIDIN-2-AMINE UTILISÉS EN TANT QU'INHIBITEURS D'HISTONE ACÉTYLTRANSFÉRASE DE C-MYC ET P300/CBP POUR LE TRAITEMENT DU CANCER申请人:GLAXOSMITHKLINE IP DEV LTD公开号:WO2019049061A1公开(公告)日:2019-03-14The invention is directed to substituted 5-(1H-benzo[d]imidazo-2-yl)- pyridin-2-amine and 5-(3H-imidazo[4,5-b]pyridin-6-yl)-pyridin-2-amine derivatives. Specifically, the invention is directed to compounds according to Formula (lb) wherein R', R2', R3', R4', Rs', R6', R7', and X1' are as defined herein; or a salt thereof including a pharmaceutically acceptable salt thereof. The compounds of the invention decrease MYC protein (c-MYC) in cells and/or inhibit p300/CBP histone acetyltransferase and can be useful in the treatment of cardiac hypertrophy, diabetes, obesity & nonalcoholic fatty liver disease, HIV, polycystic kidney disease, inflammatory diseases, ankylosing spondylitis, psoriasis, psoriatic arthritis, rheumatoid arthritis, Crohn's disease, multiple sclerosis, cancer and pre-cancerous syndromes, and diseases associated with dysregulation of Myc or inhibition of p300/CBP histone acetyltransferase. Accordingly, the invention is further directed to pharmaceutical compositions comprising a compound of the invention. The invention still further discloses methods of reducing MYC protein (c-MYC) in cells and inhibiting p300/CBP histone acetyltransferase activity, and treatment of disorders associated therewith using a compound of the invention or a pharmaceutical composition comprising a compound of the invention.本发明涉及取代的5-(1H-苯并[d]咪唑-2-基)-吡啶-2-胺和5-(3H-咪唑[4,5-b]吡啶-6-基)-吡啶-2-胺衍生物。具体而言,本发明涉及根据公式(lb)的化合物,其中R'、R2'、R3'、R4'、Rs'、R6'、R7'和X1'按本说明书中定义;或其盐,包括药用可接受的盐。本发明的化合物能够降低细胞中的MYC蛋白(c-MYC)和/或抑制p300/CBP组蛋白乙酰转移酶,可用于治疗心肌肥大、糖尿病、肥胖和非酒精性脂肪肝疾病、HIV、多囊肾疾病、炎症性疾病、强直性脊柱炎、银屑病、银屑病关节炎、类风湿性关节炎、克罗恩病、多发性硬化症、癌症和前癌症综合症,以及与Myc失调或p300/CBP组蛋白乙酰转移酶抑制相关的疾病。因此,本发明进一步涉及包含本发明化合物的药物组合物。本发明还进一步公开了使用本发明的化合物或包含本发明化合物的药物组合物,降低细胞中MYC蛋白(c-MYC)和抑制p300/CBP组蛋白乙酰转移酶活性的方法,以及治疗与之相关的疾病的方法。
-
Synthesis of thioamides via one-pot A<sup>3</sup>-coupling of alkynyl bromides, amines, and sodium sulfide
-
Method of inhibiting angiogenesis申请人:——公开号:US20040116479A1公开(公告)日:2004-06-17Compounds having the formula 1 are angiogenesis inhibitors. Also disclosed are compositions containing the compounds, methods of making the compounds, and methods of treatment using the compounds.
表征谱图
-
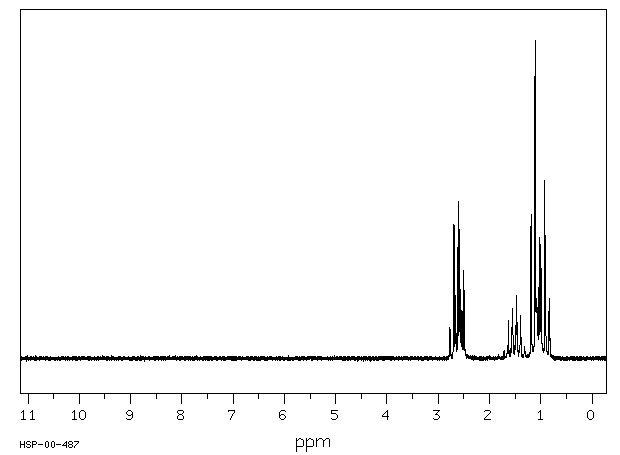
氢谱1HNMR
-
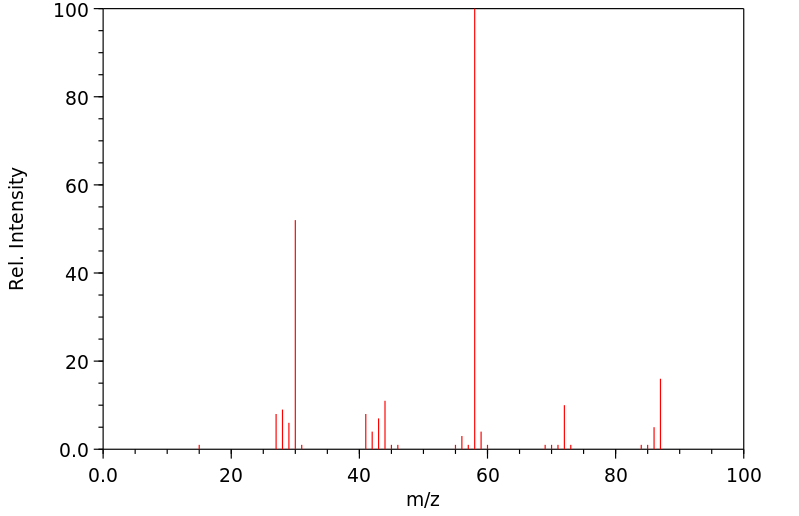
质谱MS
-
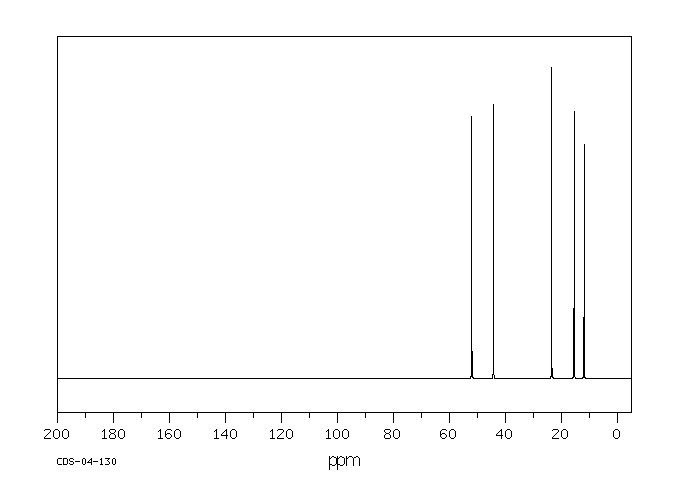
碳谱13CNMR
-
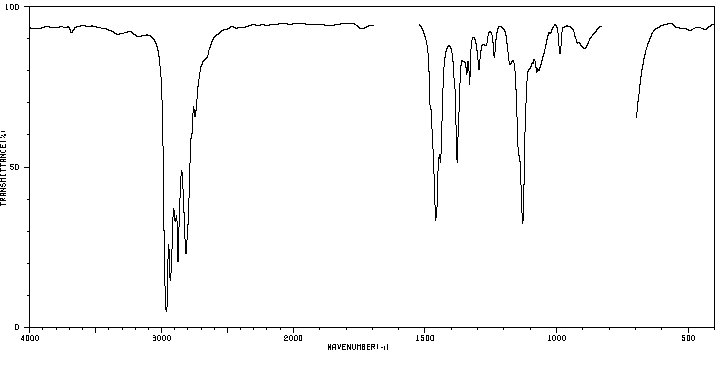
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷