3-乙基-3-己烯 | 16789-51-8
中文名称
3-乙基-3-己烯
中文别名
——
英文名称
3-ethyl-3-hexene
英文别名
3-ethylhex-3-ene
CAS
16789-51-8
化学式
C8H16
mdl
MFCD00059249
分子量
112.215
InChiKey
AUJLDZJNMXNESO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-103.01°C (estimate)
-
沸点:116 °C
-
密度:0.73
-
LogP:4.283 (est)
-
保留指数:785.9;785;786;772.2
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:8
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2901299090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— ω-gem-diethylhomoallyl chloride 82507-04-8 C8H15Cl 146.66
反应信息
-
作为反应物:参考文献:名称:还原消除中的外球和内球过程。邻位二溴烷烃的直接和间接电化学还原摘要:作为还原消除中外球和内球过程之间二分法的一个例子,研究了邻位二溴烷烃的还原。根据动力学数据分析,碳电极和芳香族阴离子自由基等外球试剂与邻位二溴烷烃根据“ET”机制发生反应,其中速率决定步骤是协同电子转移键断裂导致β-溴代烷基自由基的反应DOI:10.1021/ja00173a002
-
作为产物:参考文献:名称:在锆配合物的存在下,用二乙基镁和乙基镁卤化物对双取代的乙炔和乙烯进行选择性环金属化摘要:在存在Cp 2 ZrCl 2的情况下,用乙基卤化镁EtMgHlg(Hlg = Cl,Br)和二乙基镁Et 2 Mg对二取代的乙炔和乙烯进行催化环金属化,得到四取代的magnesacyclopenta-2,4-二烯和二取代的magnesacyclopent-2-烯。提出了形成环状不饱和有机镁化合物的可能方案,根据该方案,环金属化过程中的反应性中间体是由Cp 2 ZrCl 2,EtMgHlg,Et 2 Mg,乙炔和乙烯生成的氧化锆环戊二烯和氧化锆环戊烯。DOI:10.1134/s1070428010030097
文献信息
-
Ruthenium(<scp>ii</scp>)-catalyzed olefination <i>via</i> carbonyl reductive cross-coupling作者:Wei Wei、Xi-Jie Dai、Haining Wang、Chenchen Li、Xiaobo Yang、Chao-Jun LiDOI:10.1039/c7sc04207h日期:——Natural availability of carbonyl groups offers reductive carbonyl coupling tremendous synthetic potential for efficient olefin synthesis, yet the catalytic carbonyl cross-coupling remains largely elusive. We report herein such a reaction, mediated by hydrazine under ruthenium(II) catalysis. This method enables facile and selective cross-couplings of two unsymmetrical carbonyl compounds in either an
-
Regioselective and Stereospecific Dehydrogenative Annulation Utilizing Silylium Ion-Activated Alkenes作者:Hidekazu Arii、Yuto Yano、Kenichi Nakabayashi、Syuhei Yamaguchi、Masaki Yamamura、Kunio Mochida、Takayuki KawashimaDOI:10.1021/acs.joc.6b00793日期:2016.8.5annulation products retained the stereochemistry in cases of the reactions using internal alkenes. The use of diisopropyl(1-naphthyl)silane (2) instead of 1 also resulted in annulation to obtain the 2,3-dihydro-1-sila-1H-phenalene derivatives 6. Electrophilic aromatic substitution at the 8-position was predominant, despite the two potentially reactive positions on the naphthyl group. The steric hindrance用三苯甲基四(五氟苯基)硼酸酯(TPFPB)处理二烷基苄基硅烷(1)可获得与它们的分子内或分子间π络合物处于平衡状态的相应甲硅烷基离子,然后将其与各种烯烃进行脱氢环化反应以形成1,2,3,4-四氢-2-硅萘(4)的分离产率高达82%。硅原子上立体上更大的取代基往往会增加环状产物4的收率。在使用内部烯烃进行反应的情况下,环状产物保留了立体化学。使用二异丙基(1-萘基)硅烷(2)代替1也导致环化反应,获得2,3-二氢-1-sila-1 H-苯并菲衍生物6。尽管在萘基上有两个潜在的反应性位置,但在8位上的亲电子芳族取代仍占主导地位。萘基的空间位阻阻止了顺式烯烃向甲硅烷基离子的添加,与从1相比,这将使所需产物的收率从2大幅降低。
-
Pair-Selective Coupling of Alkynes with Alkenes on Zirconocene Complex作者:Tamotsu Takahashi、Zhenfeng Xi、Christophe J. Rousset、Noriyuki SuzukiDOI:10.1246/cl.1993.1001日期:1993.6When ethylene and alkynes such as 4-octyne and diphenylacetylene were treated with Cp2ZrBu2 highly pair selective coupling products were formed in high yields. Similarly, styrene or trimethylvinylsilane also afforded cross coupling products with alkynes on zirconocene complex.
-
Polylithiumorganic compounds - 20.作者:Adalbert Maercker、Ulrich GirreserDOI:10.1016/s0040-4020(01)85287-x日期:1994.1The reaction of various cyclic and acyclic alkynes with lithium dust (2% sodium) to form vicinal dilithioalkenes has been investigated: Aliphatic alkynes, e.g. 3-hexyne (27a), exclusively afford the corresponding (E)-dilithioalkenes, insoluble solids which are stable at room temperature and allow access to a variety of tetrasubstituted olefins in acceptable yields
-
Epoxidation of olefins by β-bromoalkoxydimethylsulfonium ylides作者:George Majetich、Joel Shimkus、Yang LiDOI:10.1016/j.tetlet.2010.10.068日期:2010.12Olefins can be converted to their respective epoxides in a one-pot procedure by dissolving the olefin in anhydrous DMSO, adding NBS to the reaction mixture to generate a beta-bromoalkoxydimethylsulfonium ylide, and then adding DBU to the reaction mixture. A large variety of alkenes were successfully epoxidized with yields largely dependent on the structure of the alkene. Most importantly, the facial selectivity of this one-pot process is the opposite of that observed when using traditional epoxidizing reagents. Electron-poor alkenes are not epoxidized under these conditions. (C) 2010 Published by Elsevier Ltd.
表征谱图
-
氢谱1HNMR
-
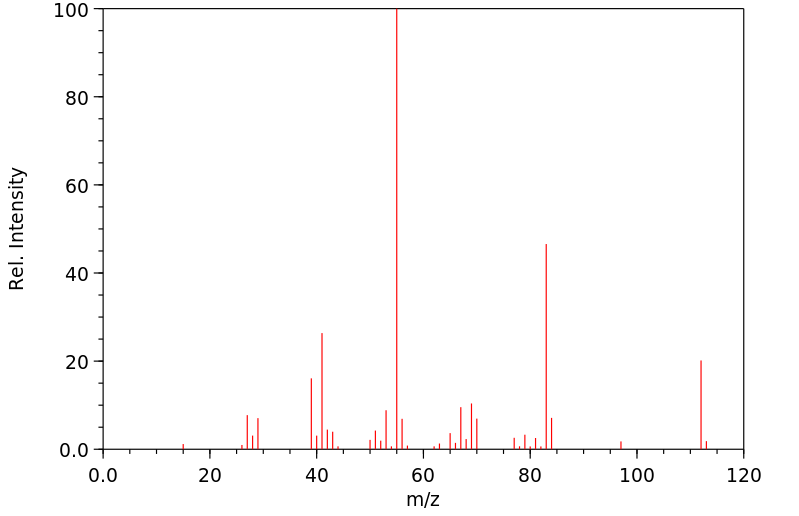
质谱MS
-
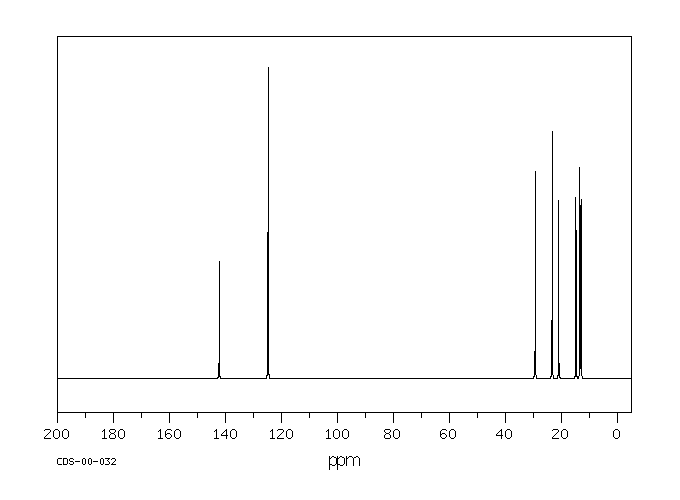
碳谱13CNMR
-
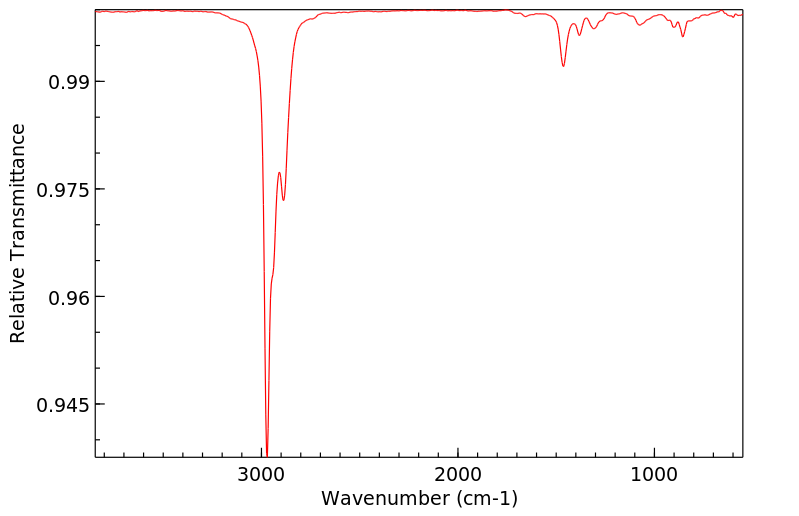
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-