(E)-1-(2,6,6-三甲基-1-环己烯-1-基)戊-1-烯-3-酮 | 63429-28-7
中文名称
(E)-1-(2,6,6-三甲基-1-环己烯-1-基)戊-1-烯-3-酮
中文别名
——
英文名称
(4E)-5-(2,6,6-trimethyl-1-cyclohexen-1-yl)-4-penten-3-one
英文别名
1E-(2,6,6-trimethyl-1-cyclohexenyl)-1-penten-3-one;1-Methyl-β-ionone;β-methylionone;1t-(2,6,6-trimethyl-cyclohex-1-enyl)-pent-1-en-3-one;1t-(2,6,6-Trimethyl-cyclohex-1-enyl)-pent-1-en-3-on;1t-(2.2.6-Trimethyl-cyclohexen-(6)-yl)-penten-(1)-on-(3);beta-Methylionone;(E)-1-(2,6,6-trimethylcyclohexen-1-yl)pent-1-en-3-one
CAS
63429-28-7;1322-71-0
化学式
C14H22O
mdl
——
分子量
206.328
InChiKey
LMWNGLDCJDIIBR-CMDGGOBGSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:281℃[at 101 325 Pa]
-
密度:0.938[at 20℃]
-
LogP:4.55 at 25℃
-
物理描述:colourless to yellow liquid
-
折光率:1.503-1.508
-
保留指数:1556.8
计算性质
-
辛醇/水分配系数(LogP):3.4
-
重原子数:15
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.64
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 beta-紫罗酮 (E)-β-ionone 79-77-6 C13H20O 192.301 —— (E)-3-(2,6,6-trimethylcyclohex-1-enyl)acrylic acid 14393-45-4 C12H18O2 194.274 —— ethyl (2E)-3-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2-propenoate 80922-54-9 C14H22O2 222.327 —— methyl β-(E)-ionol 436099-48-8 C14H24O 208.344 —— (2E)-N-methoxy-N-methyl-3-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2-propenamide 141208-06-2 C14H23NO2 237.342 —— (E)-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2-propenal S,S-acetal 121846-23-9 C15H24S2 268.488
反应信息
-
作为反应物:描述:(E)-1-(2,6,6-三甲基-1-环己烯-1-基)戊-1-烯-3-酮 在 碘 作用下, 以 neat (no solvent) 为溶剂, 反应 0.08h, 以83%的产率得到6-ethyl-1,2,3,4-tetrahydro-1,1,6-trimethylnaphthalene参考文献:名称:Selective syntheses of substituted 6-alkyl-1,1-dimethyl-1,2,3,4-tetrahydronaphthalenes摘要:Beta-ionone is cyclized to 1,1,6-trimethyl-1,2,3,4-tetrahydronaphthalene in 80-95% yield. Selective derivatization at the 5- and/or 7-positions of 6-alkyl-1,1-dimethyl-1,2,3,4-tetrahydronaphthalenes was achieved by nitration, acylation, and reduction.DOI:10.1016/s0040-4020(01)86337-7
-
作为产物:描述:参考文献:名称:NODA, PEREZ CARIDAD;DIAZ, GOMEZ MARITZA;QUINTERO, DIAZ MARIA JULIA;MAGRAN+, REV. CIENC. Y TECN., 6,(1988) N4, C. 19-23摘要:DOI:
文献信息
-
Synthesis, olfactory evaluation and determination of the absolute configuration of the β- and γ-Iralia® isomers作者:Assem Barakat、Elisabetta Brenna、Claudio Fuganti、Stefano SerraDOI:10.1016/j.tetasy.2008.09.028日期:2008.10synthesis of the methyl-ionone isomers 6–9 is described. The enantiomers of the γ-isomers 8 and 9 are prepared by enzyme-mediated resolution of the corresponding 4-hydroxy derivatives followed by reductive elimination of the hydroxy group. The absolute configuration of the latter compound is determined by chemical correlation with the known α-isomers. Since all the isomers obtained are components of the
-
Cycloalkane derivatives申请人:Green Cross Corporation公开号:US04983723A1公开(公告)日:1991-01-08A cycloalkane derivative represented by the formula (I): ##STR1## wherein A, R.sup.2, R.sup.3, R.sup.4, R.sup.5, R.sup.6, R.sup.7, Z, and n are as defined in the specification. The compounds of formula (I) exhibit activities against peptic ulcers and are useful as therapeutic and prophylactic agents for peptic ulcers.
-
POLYMER CONJUGATES FOR A CONTROLLED RELEASE OF ACTIVE MOLECULES申请人:Berthier Damien公开号:US20100098649A1公开(公告)日:2010-04-22The present invention relates to the field of perfumery. More particularly, it concerns co-polymers, derived from a maleic anhydride derivative and a ethylenic derivative, comprising at least one β-oxy or β-thio carbonyl moiety capable of liberating an active molecule such as, for example, an α,β-unsaturated ketone, aldehyde or carboxylic ester. The present invention concerns also the use of polymers or co-polymers in perfumery as well as the perfuming compositions or perfumed articles comprising the invention's compounds.
-
Retinoids and Related Compounds. Part 22. Synthesis of .BETA.-Ionone Analog Tricarbonyliron Complexes.作者:Akimori WADA、Naoko FUJIOKA、Masayoshi ITODOI:10.1248/cpb.47.171日期:——The synthesis of β-ionone analog tricarbonyliron complexes was investigated. N-Methoxy-N-methyl-(2, 6, 6-trimethyl-1-cyclohexen-1-yl)-2-propenamide (Weinreb amide), prepared from the corresponding ethyl ester and N, O-dimethylhydroxylamine hydrochloride, reacted smoothly with various organometallic reagents to afford the β-ionone analogs in good to excellent yields. Treatment of these compounds with dodecacarbonyltriiron afforded the corresponding tricarbonyliron complexes in high yields.
-
PROCESS FOR PREPARING POLYSUCCINIMIDE DERIVATIVES-BASED MICROCAPSULES申请人:Firmenich SA公开号:US20210316266A1公开(公告)日:2021-10-14Described herein is a new process for the preparation of polysuccinimide derivatives-based core-shell microcapsules. Also described are microcapsules, as well as perfuming compositions and consumer products including these microcapsules, in particular perfumed consumer products in the form of home care or personal care products.
表征谱图
-
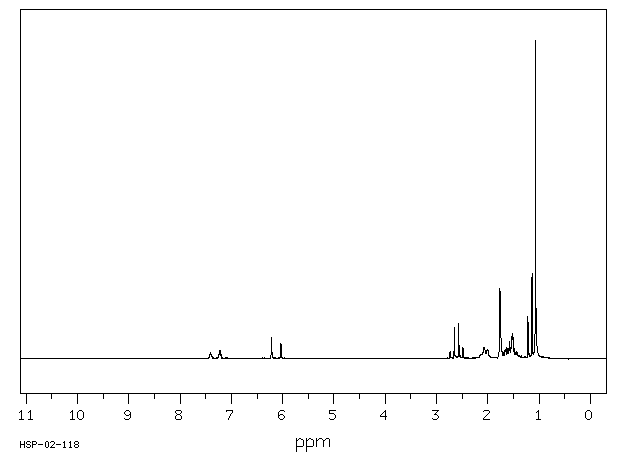
氢谱1HNMR
-
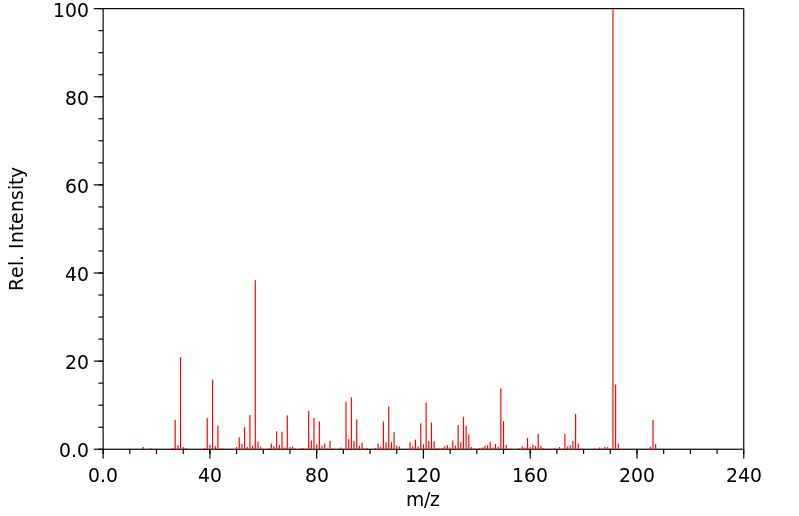
质谱MS
-
碳谱13CNMR
-
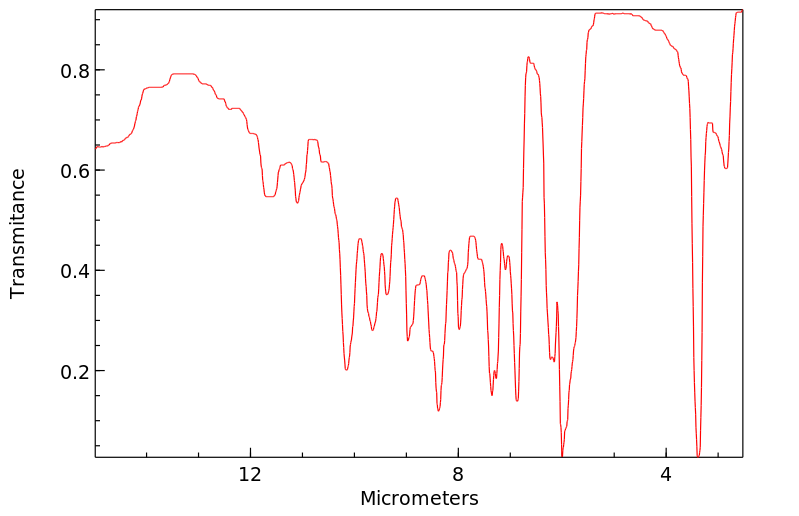
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β,6α,8α,10α,13α)-6-羟基-15-氧代黄-9(11),16-二烯-18-油酸
(3S,3aR,8aR)-3,8a-二羟基-5-异丙基-3,8-二甲基-2,3,3a,4,5,8a-六氢-1H-天青-6-酮
(2Z)-2-(羟甲基)丁-2-烯酸乙酯
(2S,4aR,6aR,7R,9S,10aS,10bR)-甲基9-(苯甲酰氧基)-2-(呋喃-3-基)-十二烷基-6a,10b-二甲基-4,10-dioxo-1H-苯并[f]异亚甲基-7-羧酸盐
(1aR,4E,7aS,8R,10aS,10bS)-8-[((二甲基氨基)甲基]-2,3,6,7,7a,8,10a,10b-八氢-1a,5-二甲基-氧杂壬酸[9,10]环癸[1,2-b]呋喃-9(1aH)-酮
(+)顺式,反式-脱落酸-d6
龙舌兰皂苷乙酯
龙脑香醇酮
龙脑烯醛
龙脑7-O-[Β-D-呋喃芹菜糖基-(1→6)]-Β-D-吡喃葡萄糖苷
龙牙楤木皂甙VII
龙吉甙元
齿孔醇
齐墩果醛
齐墩果酸苄酯
齐墩果酸甲酯
齐墩果酸溴乙酯
齐墩果酸二甲胺基乙酯
齐墩果酸乙酯
齐墩果酸3-O-alpha-L-吡喃鼠李糖基(1-3)-beta-D-吡喃木糖基(1-3)-alpha-L-吡喃鼠李糖基(1-2)-alpha-L-阿拉伯糖吡喃糖苷
齐墩果酸 beta-D-葡萄糖酯
齐墩果酸 beta-D-吡喃葡萄糖基酯
齐墩果酸 3-乙酸酯
齐墩果酸 3-O-beta-D-葡吡喃糖基 (1→2)-alpha-L-吡喃阿拉伯糖苷
齐墩果酸
齐墩果-12-烯-3b,6b-二醇
齐墩果-12-烯-3,24-二醇
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,11-二酮
齐墩果-12-烯-2α,3β,28-三醇
齐墩果-12-烯-29-酸,3,22-二羟基-11-羰基-,g-内酯,(3b,20b,22b)-
齐墩果-12-烯-28-酸,3-[(6-脱氧-4-O-b-D-吡喃木糖基-a-L-吡喃鼠李糖基)氧代]-,(3b)-(9CI)
齐墩果-12-烯-28-酸,3,7-二羰基-(9CI)
齐墩果-12-烯-28-酸,3,21,29-三羟基-,g-内酯,(3b,20b,21b)-(9CI)
鼠特灵
鼠尾草酸醌
鼠尾草酸
鼠尾草酚酮
鼠尾草苦内脂
黑蚁素
黑蔓醇酯B
黑蔓醇酯A
黑蔓酮酯D
黑海常春藤皂苷A1
黑檀醇
黑果茜草萜 B
黑五味子酸
黏黴酮
黏帚霉酸