(E)-prop-1-en-1-ylcyclohexane | 2114-41-2
中文名称
——
中文别名
——
英文名称
(E)-prop-1-en-1-ylcyclohexane
英文别名
(E)-1-cyclohexyl-1-propene;(E)-1-cyclohexylprop-1-ene;(E)-prop-1-enylcyclohexane;E-1-cyclohexylprop-1-ene;(E)-1-cyclohexylpropene;trans-Propenyl-cyclohexan;Cyclohexane, 1-propenyl-;[(E)-prop-1-enyl]cyclohexane
CAS
2114-41-2
化学式
C9H16
mdl
——
分子量
124.226
InChiKey
IAHIMVFWYADCJJ-QHHAFSJGSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
保留指数:923.3
计算性质
-
辛醇/水分配系数(LogP):3.8
-
重原子数:9
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.78
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
上下游信息
反应信息
-
作为反应物:参考文献:名称:Allenic 和 Acetylenic 衍生物的区域和立体控制合成。有机钛和硼试剂摘要:由 1-烷基丙炔衍生的炔丙基钛试剂与醛缩合生成 α-丙炔醇,而由 1-烷基 1-1-丁炔衍生物生成的丙炔基钛试剂生成具有高区域选择性和立体选择性的苏式-β-炔醇。反应过程由起始炔烃的取代模式决定。还研究了金属化 1,3-双(三烷基甲硅烷基)丙炔或(三烷基甲硅烷基)乙腈与醛的类似反应。DOI:10.1246/bcsj.57.2768
-
作为产物:描述:(R*,R*)-2-Diphenylphosphinoyl-1-cyclohexylpropan-1-ol 在 sodium hydride 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 以80.2%的产率得到(E)-prop-1-en-1-ylcyclohexane参考文献:名称:Buss, Antony D.; Warren, Stuart, Journal of the Chemical Society. Perkin transactions I, 1985, p. 2307 - 2326摘要:DOI:
文献信息
-
Hindered organoboron groups in organic chemistry. 25. The condensation of aliphatic aldehydes with dimesitylboryl stabilised carbanions to give alkenes.作者:Andrew Pelter、Keith Smith、Said M.A. ElgendyDOI:10.1016/s0040-4020(01)87983-7日期:1993.8In the presence of protic acids the condensation of aliphatic aldehydes with dimesitylboryl stabilised carbanions results in alkenes. In the presence of strong acids such as HCl or CF3SO3H, the products contain > 90% of E-alkenes in all cases tried. When acetic acid is used, then Z-alkenes may result predominantly, particularly in the cases of RsCHO and RtCHO. HX HCI, CF3SO3H gives E - alkenes in在质子酸的存在下,脂族醛与二聚三苯甲基稳定的碳负离子的缩合会生成烯烃。在强酸(例如HCl或CF 3 SO 3 H)的存在下,在所有尝试的情况下,产品均含有> 90%的E-烯烃。当使用乙酸时,则可能主要产生Z-烯烃,特别是在R s CHO和R t CHO的情况下。在所有情况下,HXHCl,CF 3 SO 3 H均会生成E-烯烃。HXCH 3 CO 2 H,使得主要ž -烯烃当Rř秒,R叔。
-
Hindered organoboron groups in organic synthesis. 14. stereoselective synthesis of alkenes by the boron-wittig reaction using aliphatic aldehydes作者:Andrew Pelter、Keith Smith、Said Elgendy、Martin RowlandsDOI:10.1016/s0040-4039(01)93821-3日期:1989.1In the presence of HX, carbanions Mes2BCHLiR1 react with aliphatic aldehydes to give alkenes. The stereochemistry of the product alkene depends upon the nature of HX.在HX存在下,碳负离子Mes 2 BCHLiR 1与脂族醛反应生成烯烃。产物烯烃的立体化学取决于HX的性质。
-
Iron-Catalyzed Tunable and Site-Selective Olefin Transposition作者:Xiaolong Yu、Haonan Zhao、Ping Li、Ming Joo KohDOI:10.1021/jacs.0c08631日期:2020.10.21earth-abundant iron-based complex, a base and a boryl compound promote efficient and controllable alkene transposition. Mechanistic investigations reveal that these processes likely involve in situ formation of an iron-hydride species which promotes olefin isomerization through sequential olefin insertion/β-hydride elimination. Through this strategy, regiodivergent access to different products from
-
Highly chemo- and stereoselective Fe-catalyzed alkenylation of organomanganese reagents作者:Gérard Cahiez、Sophie MarquaisDOI:10.1016/0040-4039(96)00116-5日期:1996.3Organomanganese chlorides react with alkenyl iodides, bromides and chlorides in the presence of 3% Fe(acac)3. The reaction takes place under very mild conditions (THF-NMP, rt, 1h) to afford the substituted olefin in excellent yields with a high stereo- and chemoselectivity. Thus an unprotected keto alkenyl chloride selectively gives the corresponding keto olefin. From a preparative point of view, this
-
Visible‐Light Controlled Divergent Catalysis Using a Bench‐Stable Cobalt(I) Hydride Complex作者:Enrico Bergamaschi、Frédéric Beltran、Christopher J. TeskeyDOI:10.1002/chem.202000410日期:2020.4.21the ability to switch the actual function of the catalyst and resulting products. Here we report such an example of multi-dimensional catalysis. Featuring an easily prepared, bench-stable cobalt(I) hydride complex in conjunction with pinacolborane, we can switch the reaction outcome between two widely employed transformations, olefin migration and hydroboration, with visible light as the trigger.
表征谱图
-
氢谱1HNMR
-
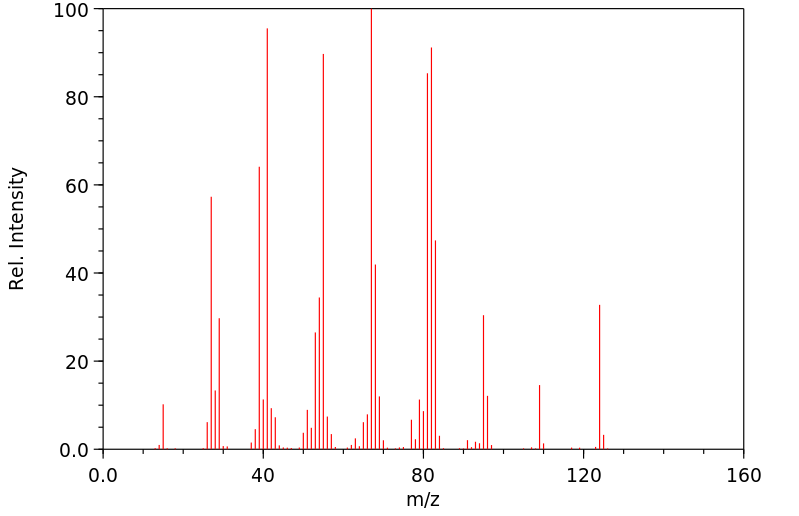
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-