二螺[5,1,5,1]十四烷-7,14-二酮 | 950-21-0
中文名称
二螺[5,1,5,1]十四烷-7,14-二酮
中文别名
——
英文名称
dispiro[5.1.5.1]tetradecane-7,14-dione
英文别名
Dispiro<5.1.5.1>tetradecan-7,14-dion;Dispiro<5.1.5.1>tetradecandion-(7,14);dispiro[5.1.58.16]tetradecane-7,14-dione
CAS
950-21-0
化学式
C14H20O2
mdl
——
分子量
220.312
InChiKey
NQCABGRZRRCWPC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:165-165 °C
-
沸点:390.0±17.0 °C(Predicted)
-
密度:1.11±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.8
-
重原子数:16
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.86
-
拓扑面积:34.1
-
氢给体数:0
-
氢受体数:2
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 14-硫氧代-二螺[5.1.5.1]-7-十四酮 1,4-thioxodispiro[5.1.58.16]tetradecan-7-one 22502-48-3 C14H20OS 236.378 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 14-硫氧代-二螺[5.1.5.1]-7-十四酮 1,4-thioxodispiro[5.1.58.16]tetradecan-7-one 22502-48-3 C14H20OS 236.378
反应信息
-
作为反应物:描述:二螺[5,1,5,1]十四烷-7,14-二酮 在 tetrabutylammonium tetrafluoroborate 五氯化磷 作用下, 以 四氢呋喃 为溶剂, 反应 2.5h, 生成 1',3'-dichlorodispiro(cyclohexane-1,2'-bicyclo<1.1.0>butane-4',1''-cyclohexane)参考文献:名称:Strelow, Thomas; Voss, Juergen; Adiwidjaja, Gunadi, Journal of Chemical Research, Miniprint, 1989, # 5, p. 1148 - 1161摘要:DOI:
-
作为产物:描述:参考文献:名称:Regitz,M.; Rueter,J., Chemische Berichte, 1969, vol. 102, p. 3877 - 3890摘要:DOI:
文献信息
-
Alkoxide-catalyzed ring expansion of 1,3-cyclobutanediones with aldehydes作者:Mizuki Yamazaki、Tomoyuki Yoshimura、Jun-ichi MatsuoDOI:10.1016/j.tetlet.2020.151804日期:2021.21,3-Cyclobutanediones (dialkyl ketene dimer) reacted with various aromatic aldehydes to give six-membered cyclic β-keto esters by using a catalytic amount of potassium ethoxide. Effects of alkoxide catalysts and spiro cyclic groups at the 2,4-positions of 1,3-cyclobutanediones were investigated.
-
Effect of Spiro Ring Size on the Photochemical Ring Expansion of Dispiro Substituted Cyclobutane-1,3-diones作者:Koji Kimura、Masakazu Takamura、Shigeru Koshibe、Masayoshi Juro、Yoshihiro Fukuda、Yoshinobu OdairaDOI:10.1246/bcsj.49.741日期:1976.3The photochemical ring expansion of dispiro substituted cyclobutane-1,3-diones in methanol has been investigated. The formation of ring expansion product was strongly dependent on the spiro ring size of dispiro substituent. The ring expanded acetal was obtained in a low yield when the spiro ring of dispiro substituted cyclobutane-1,3-diones was a five- or seven-membered ring. This is the first example
-
Ring strain release as a strategy to enable the singlet state photodecarbonylation of crystalline 1,4-cyclobutanediones作者:Gregory Kuzmanich、Miguel A. Garcia-GaribayDOI:10.1002/poc.1902日期:2011.10is essential to slow down the combination of the intermediate acyl–alkyl biradical back to the starting ketone. Relatively long triplet acyl–alkyl biradical lifetimes give a chance for the loss of CO to occur. Looking for additional strategies to generate transient biradicals in solids, we studied the solid state photochemistry of four aliphatic, dispiro‐substituted 1,4‐cyclobutandiones (1a–d) that挑战大多数化学家的直觉,利用环状酮的光脱羰基化可以在固态下可靠地生成高反应性的二烷基双自由基。但是,已经表明,在前体的α-碳上具有共振解离能力的自由基稳定基团是促进α裂解反应所必需的,并且三重态反应性对于减慢中间酰基的结合是必不可少的。烷基双自由基回到起始酮。相对较长的三重态酰基-烷基双自由基寿命为发生CO损失提供了机会。为了寻找在固体中生成瞬态双基的其他策略,我们研究了预期从单线态反应的四种脂族,双螺取代的1,4-环丁二酮(1a–d)的固态光化学。我们假设小环羰基释放出环应变将使反向酰基-烷基组合不利,从而使CO的损失得以有效且不可逆地发生。我们在这里报告在溶液,块状(粉末)晶体和纳米晶体光化学中进行的研究结果。我们最近表明,对二螺环己基-1,3-环丁二酮1c的激发导致中间羟基烯丙基的捕获,其半衰期约为42分钟。我们对其他三种结晶衍生物的研究表明,尽管它们均能有效反应,但羟烯丙基的超长寿
-
The reaction of 2,2,4,4-tetramethyl-1,3-cyclobutanedione with<i>o</i>-aminophenols,<i>o</i>-aminothiophenol and with aliphatic 2- and 3-hydroxy- and -mercaptoamines作者:Siegfried LinkeDOI:10.1002/jhet.5570100507日期:1973.10Studies of the reaction of the dione dimer of dimethylketene (1) with a series of o-aminophenols and with o-aminothiophenol have been carried out. These reactions give 2-[2-(2,4-dimethyl-3-oxopentyl)]benzoxazoles and -benzothiazole, respectively. Aliphatic 2- and 3-hydroxy- and 2- and 3-mercaptoamines yield 2-substituted 2-oxazolines, 5,6-dihydro-4H-1,3-oxazines, 2-thiazolines and 5,6-dihydro-4H-1
-
METHOD FOR PRODUCING CYCLOBUTANEDIOL COMPOUND申请人:JNC CORPORATION公开号:US20210300847A1公开(公告)日:2021-09-30Provided is a process in which a cyclobutanediol compound having a high cis:trans ratio can be stably obtained. The cyclobutanediol compound having a cis:trans ratio of 1.5:1 to 5000:1 is produced by using at least one compound selected from a group consisting of a cyclobutanedione compound, a cyclobutanketol compound, and a cyclobutanediol compound as a raw material, and performing a catalytic hydrogenation reaction and an isomerization reaction in the cyclobutanediol compound in a solid phase state in the presence of a metal catalyst without adding a solvent.
表征谱图
-
氢谱1HNMR
-
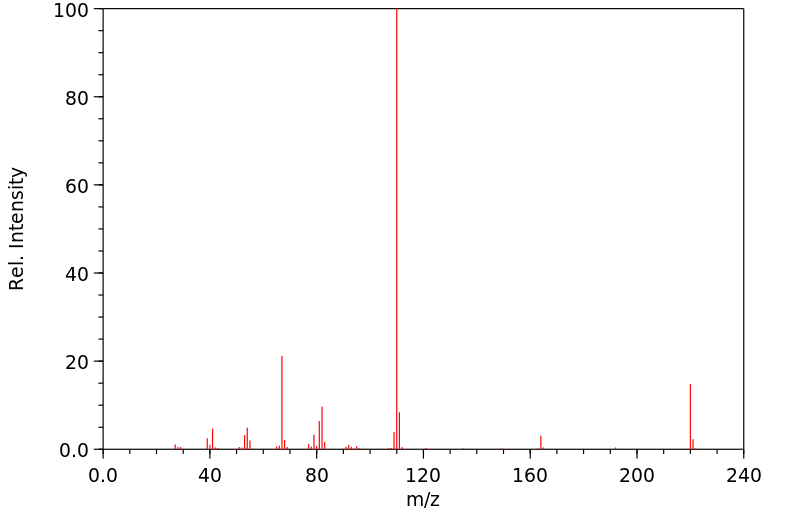
质谱MS
-
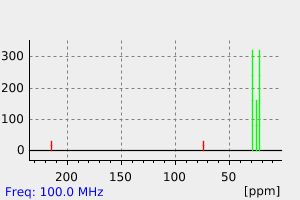
碳谱13CNMR
-
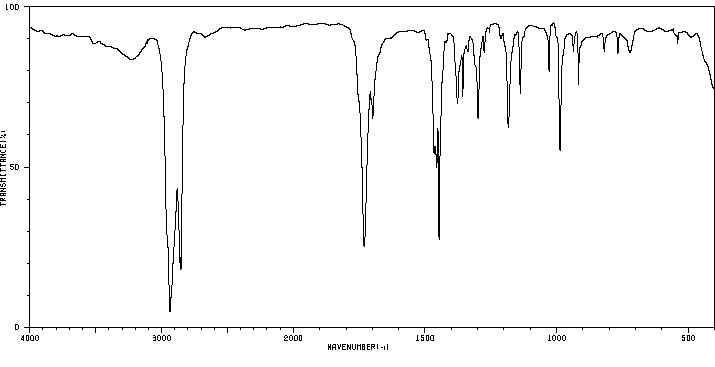
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷