1-(4-氯苯基)-1-环丁羧酸 | 50921-39-6
物质功能分类
中文名称
1-(4-氯苯基)-1-环丁羧酸
中文别名
1-(4-氯苯基)-环丁烷羧酸
英文名称
1-(4-chlorophenyl)cyclobutane-1-carboxylic acid
英文别名
1-(4-chlorophenyl)cyclobutanecarboxylic acid;1-(4-chlorophenyl)-1-cyclobutanecarboxylic acid
CAS
50921-39-6
化学式
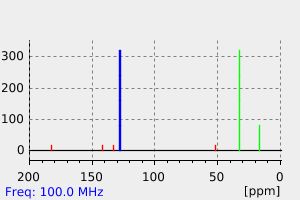
C11H11ClO2
mdl
MFCD00001324
分子量
210.66
InChiKey
XYSRHOKREWGGFE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:80-90 °C
-
沸点:302.49°C (rough estimate)
-
密度:1.1742 (rough estimate)
-
稳定性/保质期:
遵照规定使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):2.9
-
重原子数:14
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.363
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险品标志:Xi
-
安全说明:S24/25
-
危险类别码:R36/37/38
-
海关编码:2916399090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
SDS
| Name: | 1-(4-Chlorophenyl)-1-Cyclobutane- Carboxylic Acid 94% Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 50921-39-6 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 50921-39-6 | 1-(4-Chlorophenyl)-1-Cyclobutane- Carb | 94% | 256-847-3 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 50921-39-6: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: beige to light purple
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 80.00 - 82.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
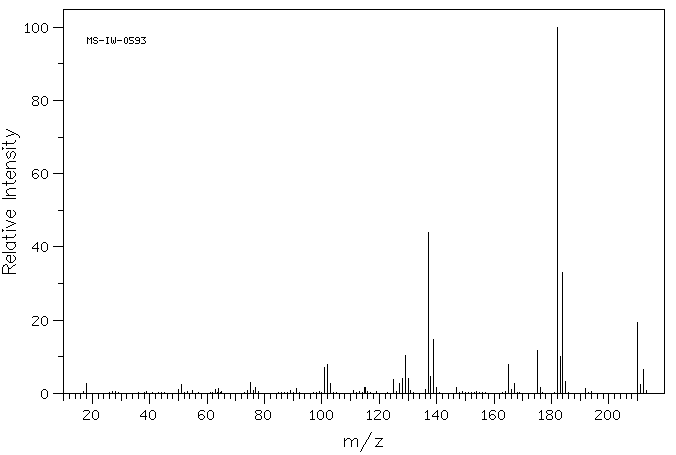
Molecular Formula: C11H11ClO2
Molecular Weight: 210.66
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Hydrogen chloride, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 50921-39-6 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1-(4-Chlorophenyl)-1-Cyclobutane- Carboxylic Acid - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 50921-39-6: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 50921-39-6 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 50921-39-6 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-(4-氯苯基)-1-氰基环丁烷 1-(4-chlorophenyl)cyclobutanecarbonitrile 28049-61-8 C11H10ClN 191.66 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-苯基环丁基甲酸 1-(phenyl)cyclobutane-1-carboxylic acid 37828-19-6 C11H12O2 176.215 —— Methyl 1-(4-chlorophenyl)cyclobutane-1-carboxylate 918873-02-6 C7H3FNO4Pol 224.68 —— 1-(4-chlorophenyl)cyclobutylmethanol 50921-41-0 C11H13ClO 196.677 l-(4-氯-苯基)-环丁烷羰酰氯 l-(4-chloro-phenyl)-cyclobutanecarbonyl chloride 89421-95-4 C11H10Cl2O 229.106 —— 3'-phenoxybenzyl 1-(4-chlorophenyl)cyclobutanecarboxylate 66130-58-3 C24H21ClO3 392.882 —— 1-(1-(4-chlorophenyl)cyclobutyl)-2-diazoethanone 1309195-50-3 C12H11ClN2O 234.685
反应信息
-
作为反应物:描述:参考文献:名称:Substituted alkanoic acids摘要:该发明涉及一般式(I)的生理活性化合物: 1 其中:- 2 表示(i)饱和的3至6成员碳环,可选地由一个或多个烷基取代,(ii)吲哚基或(iii)饱和的4至6成员杂环环; R 1 表示R 3 Z 1 -Het-或R 4 N(R 5 )—C(═O)—NH—Ar 1 —; L 1 表示一个—R 6 —R 7 —连接; R 2 表示氢、卤素、低烷基或低烷氧基; L 2 表示一个烷基连接; Y为羧基或酸生物同位素; 以及这些化合物及其相应的N-氧化物或前药,以及这些化合物及其相应的N-氧化物或前药的药学上可接受的盐和溶剂。 这些化合物具有有价值的药理特性,特别是调节VCAM-1和纤维连接蛋白与整合素VLA-4(&agr;4&bgr;1)相互作用的能力。公开号:US20040006056A1
-
作为产物:描述:参考文献:名称:极性取代基对1-芳基环丁基甲基格氏试剂环断裂重排的影响摘要:已经确定了1-苯基环丁基甲基镁氯化物及其对甲基和对氯类似物的环断裂重排的动力学。一阶速率常数由Hammett方程关联,ϱ = -0.47。该结果与具有显着极性特征的具有环状过渡态的协调机制一致,尽管极性对反应物和产物的稳定性也有贡献。苯基本身使反应减慢了0.031倍,这主要是根据过渡态的空间不稳定来解释的。DOI:10.1016/0022-328x(93)80060-o
文献信息
-
Substituted alkyl amido piperidines申请人:Synaptic Pharmaceutical Corporation公开号:US20040186103A1公开(公告)日:2004-09-23This invention is directed to compounds which are selective antagonists for melanin concentrating hormone-1 (MCH1) receptors. The invention provides a pharmaceutical composition comprising a therapeutically effective amount of the compound of the invention and a pharmaceutically acceptable carrier. This invention provides a pharmaceutical composition made by combining a therapeutically effective amount of the compound of this invention and a pharmaceutically acceptable carrier. This invention further provides a process for making a pharmaceutical composition comprising combining a therapeutically effective amount of the compound of the invention and a pharmaceutically acceptable carrier. This invention also provides a method of reducing the body mass of a subject which comprises administering to the subject an amount of a compound of the invention effective to reduce the body mass of the subject. This invention further provides a method of treating a subject suffering from depression and/or anxiety which comprises administering to the subject an amount of a compound of the invention effective to treat the subject's depression and/or anxiety.这项发明涉及选择性拮抗黑色素浓缩激素-1(MCH1)受体的化合物。该发明提供了一种包括该发明化合物的治疗有效量和药学上可接受的载体的药物组合物。该发明提供了一种由结合本发明化合物的治疗有效量和药学上可接受的载体制成的药物组合物。该发明还提供了一种制备药物组合物的方法,包括结合本发明化合物的治疗有效量和药学上可接受的载体。该发明还提供了一种减少受试者体重的方法,包括向受试者施用本发明化合物的有效量以减少受试者的体重。该发明还提供了一种治疗患有抑郁症和/或焦虑症的受试者的方法,包括向受试者施用本发明化合物的有效量以治疗受试者的抑郁症和/或焦虑症。
-
Automated Synthesis and Purification of Amides: Exploitation of Automated Solid Phase Extraction in Organic Synthesis作者:R. Michael Lawrence、Scott A. Biller、Olga M. Fryszman、Michael A. PossDOI:10.1055/s-1997-1232日期:1997.5Automated parallel synthesis of small organic molecules, either as single entities or as mixtures, offers the potential for the rapid optimization of physical and biological properties of a molecule. Currently, emphasis has been placed on solid phase synthesis technology to accomplish the rapid preparation of large numbers of molecules. Automated solution phase synthesis is an alternative approach which has the advantages of having shorter development times and being more amenable to scaleup. Utilizing commercially available liquid handlers for reaction setup and exploiting automated solid phase extraction for product purification, a procedure has been developed to prepare and purify up to 100 amide analogs, simultaneously. Both carbodiimide mediated couplings and p-nitrophenyl ester displacements have been carried out using this procedure. Product amides having overall neutral or basic character have been prepared in good yield and with good to excellent purities.
-
New oxybenzamide derivatives useful for inhibiting factor Xa or VIIa申请人:——公开号:US20020198195A1公开(公告)日:2002-12-26The present invention relates to compounds comprising the following formula: R 0 —Q—X—Q′—W—U—V—G—M (I) These compounds are useful as pharmacologically active compounds. They exhibit an antithrombotic effect and are suitable, for example, for the therapy and prophylaxis of cardiovascular disorders such as thromboembolic diseases or restenoses. These compounds are reversible inhibitors of the blood clotting enzymes factor Xa (FXa) and/or factor VIIa (FVIIa), and can generally be used to treat, prevent, or cure conditions in which an undesired activity of factor Xa and/or factor VIIa is present, or where inhibition of factor Xa and/or factor VIIa is intended. The invention further relates to processes for the preparation of these compounds, methods of their use (e.g., as active ingredients in pharmaceuticals), and pharmaceutical preparations comprising them.本发明涉及包含以下公式的化合物: R 0 —Q—X—Q′—W—U—V—G—M (I) 这些化合物作为药物活性化合物是有用的。它们展现出抗血栓作用,并适用于例如治疗和预防心血管疾病,如血栓栓塞性疾病或再狭窄。这些化合物是血液凝固酶因子Xa(FXa)和/或因子VIIa(FVIIa)的可逆性抑制剂,通常可用于治疗、预防或治愈存在因子Xa和/或因子VIIa不期望活性的情况,或旨在抑制因子Xa和/或因子VIIa的情况。本发明还涉及制备这些化合物的方法、它们的使用方法(例如,作为药品中的活性成分)以及包含它们的药物制剂。
-
[EN] ORGANIC COMPOUNDS<br/>[FR] COMPOSES ORGANIQUES申请人:SPEEDEL EXPERIMENTA AG公开号:WO2005090304A1公开(公告)日:2005-09-29The invention relates to novel amino alcohols of the general formula (I) where X, R1, R2, R3, R4, R5 and R6 are each as defined in detail in the description, to a process for their preparation and to the use of these compounds as medicines, in particular as renin inhibitors.
-
Nucleophilic (Radio)Fluorination of Redox-Active Esters via Radical-Polar Crossover Enabled by Photoredox Catalysis作者:Eric W. Webb、John B. Park、Erin L. Cole、David J. Donnelly、Samuel J. Bonacorsi、William R. Ewing、Abigail G. DoyleDOI:10.1021/jacs.0c03125日期:2020.5.20redox-neutral method for nucleophilic fluorination of N-hydroxyphthalimide esters using an Ir photocatalyst under visible light irradiation. The method provides access to a broad range of aliphatic fluorides, including primary, secondary, and tertiary benzylic fluorides as well as unactivated tertiary fluorides, that are typically inaccessible by nucleophilic fluorination due to competing elimination
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
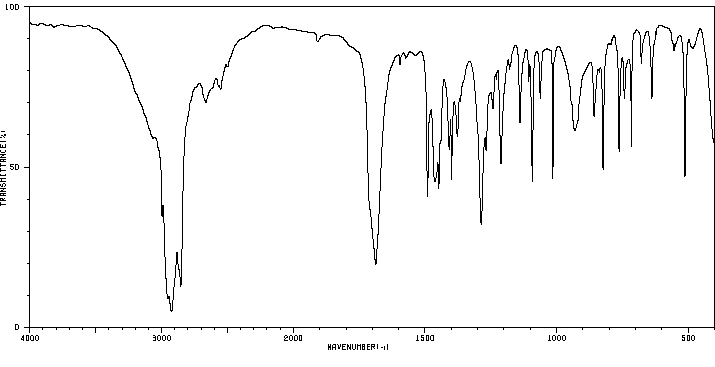
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫