N-(3-acetyl-2-nitrophenyl)acetamide | 92642-18-7
中文名称
——
中文别名
——
英文名称
N-(3-acetyl-2-nitrophenyl)acetamide
英文别名
3-acetamido-2-nitroacetophenone;acetic acid-(3-acetyl-2-nitro-anilide);1-(2-Nitro-3-acetamino-phenyl)-aethanon-(1);Essigsaeure-(3-acetyl-2-nitro-anilid)
CAS
92642-18-7
化学式
C10H10N2O4
mdl
——
分子量
222.2
InChiKey
ANTGAGQFTOTKDZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.2
-
重原子数:16
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:92
-
氢给体数:1
-
氢受体数:4
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-(3-氨基-2-硝基苯基)乙酮 1-(3-amino-2-nitrophenyl)ethanone 10202-87-6 C8H8N2O3 180.163 —— 1-(2,3-bis-acetylamino-phenyl)-ethanone —— C12H14N2O3 234.255
反应信息
-
作为反应物:描述:N-(3-acetyl-2-nitrophenyl)acetamide 在 盐酸 、 palladium 10% on activated carbon 、 氢气 作用下, 以 甲醇 、 乙醇 、 水 为溶剂, 反应 3.0h, 生成 1-(2,3-二氨基苯基)乙酮参考文献:名称:酮甲基或芳基通过C ?直接交换成另一个芳基 铑螯合辅助的C键活化摘要:交换:在Rh I络合物存在下,将市售的喹啉酮衍生物(1或2,参见方案)与芳基硼酸反应,以中等至良好的收率得到芳基(quinolin-8-yl)methanone产品3 提出了一种机制,该机制涉及在CuI存在下用O 2将Rh I原位氧化为Rh III。DOI:10.1002/anie.201206693
-
作为产物:描述:参考文献:名称:酮甲基或芳基通过C ?直接交换成另一个芳基 铑螯合辅助的C键活化摘要:交换:在Rh I络合物存在下,将市售的喹啉酮衍生物(1或2,参见方案)与芳基硼酸反应,以中等至良好的收率得到芳基(quinolin-8-yl)methanone产品3 提出了一种机制,该机制涉及在CuI存在下用O 2将Rh I原位氧化为Rh III。DOI:10.1002/anie.201206693
文献信息
-
Heterocyclic rearrangements. Part VI. Rearrangements of 4-acyl- and 4-iminoalkyl-benzofuroxans: new syntheses of the anthranil and indazole ring systems作者:A. J. Boulton、P. B. Ghosh、A. R. KatritzkyDOI:10.1039/j29660001011日期:——4-Acyl- and 4-iminoalkyl-benzofuroxans, on preparation by nitrogen elimination from the corresponding 3-substituted 2-nitrophenyl azides, rearrange spontaneously to the anthranil and indazole ring systems. Attempts to prepare 4-alkenylbenzofuroxans failed. In 3-chloro-2-nitro- and 3-methoxy-2-nitro-benzaldehydes, the nitro-group is displaced by nucleophils in preference to the methoxy-group of chlorine
-
Site-specific labelling of caged atp with deuterium or 18oxygen作者:John E. T. Corrie、Gordon P. ReidDOI:10.1002/jlcr.2580360312日期:1995.3[3-D]2-Nitroacetophenone and [alcohol-18O]-1-(2-nitrophenyl)ethyl alcohol were prepared and used to synthesise labelled P3-l-(2-nitrophenyl)ethyl esters of ATP (“caged ATP”) with isotope present either as deuterium on the 3-position of the nitrosubstituted ring or as 18oxygen in the bridging position between the terminal phosphate and the nitrophenylethyl group. The availability of the deuterated compounds enabled complete assignment of their 1H NMR spectra.
-
Simpson et al., Journal of the Chemical Society, 1945, p. 646,655作者:Simpson et al.DOI:——日期:——
-
CINNOLINES. I. SYNTHESIS OF AMINOACETOPHENONES AND AMINOPROPIOPHENONES<sup>1</sup>作者:NELSON J. LEONARD、SAMUEL N. BOYDDOI:10.1021/jo01174a018日期:1946.7
-
Substituted 1,10-Phenanthrolines. XI. Aza Derivatives<sup>1</sup>作者:Francis H. Case、James A. BrennanDOI:10.1021/ja01532a046日期:1959.12
表征谱图
-
氢谱1HNMR
-
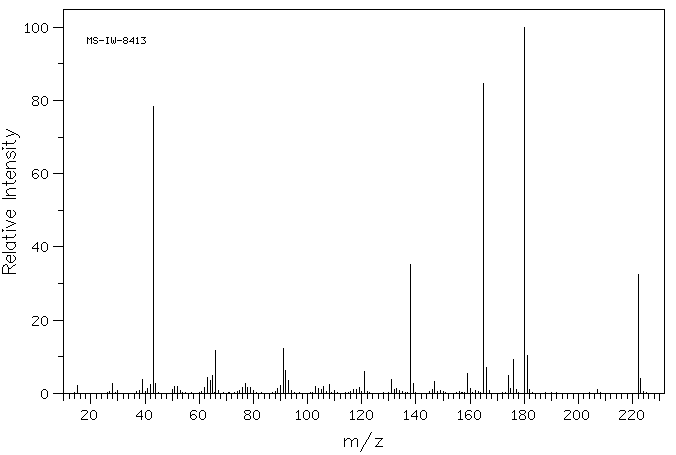
质谱MS
-
碳谱13CNMR
-
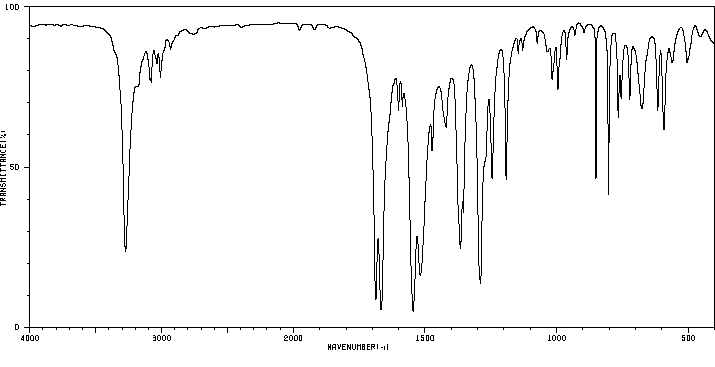
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷