3,5-二(三氟甲基)苯丙酮 | 85068-34-4
中文名称
3,5-二(三氟甲基)苯丙酮
中文别名
3,5-双(三氟甲基)苯丙酮;3,5-双三氟甲基苯丙酮
英文名称
1-[3,5-bis(trifluoromethyl)phenyl]-propan-1-one
英文别名
1-(3,5-bis(trifluoromethyl)phenyl)propan-1-one;1-[3,5-bis(trifluoromethyl)phenyl]propan-1-one;3',5'-bis(trifluoromethyl)propiophenone;3',5'-bistrifluoromethylpropiophenone;1-[3,5-bis(trifluoromethyl)phenyl]propanone;3',5'-bis-trifluoromethylpropiophenone
CAS
85068-34-4
化学式
C11H8F6O
mdl
MFCD00009911
分子量
270.174
InChiKey
VLRWCHKOSBUGMB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:72°C 7mm
-
密度:1.379 g/mL at 25 °C(lit.)
-
闪点:169 °F
-
稳定性/保质期:
按规定使用和贮存的物质不会分解,并能避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):3.8
-
重原子数:18
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.363
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:7
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
危险类别码:R36/38
-
WGK Germany:3
-
海关编码:2914700090
-
安全说明:S26,S36
-
危险性防范说明:P305+P351+P338
-
危险性描述:H227,H315,H319,H335
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3,5-双三氟甲基苯甲醛 3,5-Bis(trifluoromethyl)benzaldehyde 401-95-6 C9H4F6O 242.121 1-[3,5-双(三氟甲基)苯基]丙醇 (-)-1-(3,5-bis(trifluoromethyl)phenyl)propan-1-ol 742097-70-7 C11H10F6O 272.19 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-[3,5-双(三氟甲基)苯基]丙醇 (-)-1-(3,5-bis(trifluoromethyl)phenyl)propan-1-ol 742097-70-7 C11H10F6O 272.19 —— (+)-(R)-1-(3,5-bis(trifluoromethyl)phenyl)propan-1-ol —— C11H10F6O 272.19 N-(3-氟苯基)-beta-丙氨酸 1-[3,5-bis(trifluoromethyl)phenyl]propylamine 685503-45-1 C11H11F6N 271.205
反应信息
-
作为反应物:描述:3,5-二(三氟甲基)苯丙酮 在 2-氨甲基吡啶 、 五羰基溴化锰(I) 、 potassium tert-butylate 、 氢气 作用下, 以 四氢呋喃 为溶剂, 120.0 ℃ 、3.0 MPa 条件下, 反应 12.0h, 以23%的产率得到1-[3,5-双(三氟甲基)苯基]丙醇参考文献:名称:锰催化的酮均相氢化和α , β -不饱和羧酸衍生物的共轭还原:一种化学选择性、稳健且不含膦的原位方案摘要:我们传达了一种用户友好且无需手套箱的催化协议,用于酮和酯和腈的共轭 C C键的锰催化氢化。相应的催化剂很容易由特权 [Mn(CO) 5 Br] 前体和廉价的 2-吡啶甲胺原位组装。催化转化在t- BuOK存在下进行,从而以良好至极好的收率获得相应的氢化产物。所描述的系统提供了对仲醇和饱和酯的快速和原子效率的访问,避免了使用氧敏感和昂贵的基于膦的配体。DOI:10.1016/j.apcata.2021.118280
-
作为产物:描述:参考文献:名称:Thiourea inhibitors of herpes viruses. Part 2: N-Benzyl-N′-arylthiourea inhibitors of CMV摘要:A series of highly potent thiourea inhibitors of cytomegalovirus (CMV) with improved stability properties was prepared and evaluated. Compound 29 inhibited the virus in cultured HFF cells with IC50 of 0.2 nM. (C) 2004 Published by Elsevier Ltd.DOI:10.1016/j.bmcl.2004.04.093
文献信息
-
[EN] NOVEL NEUROKININ 1 RECEPTOR ANTAGONIST COMPOUNDS<br/>[FR] NOUVEAUX COMPOSÉS ANTAGONISTES DU RÉCEPTEUR DE LA NEUROKININE 1申请人:LEO PHARMA AS公开号:WO2013124286A1公开(公告)日:2013-08-29The present invention relates to a compound according to formula (A) wherein n is 1 or 2; R1 and R2 are independently hydrogen, C1-4 alkyl, C1-4 haloalkyl, C1-4 alkoxy, CD3 or halogen; R3 is hydrogen, C(=O)OR7 or C1-4 alkyl optionally substituted with hydroxy or NR8R9; R4 is hydrogen or oxo; R5 and R6 are independently hydrogen, hydroxy, NR8R9, C( =O)R7, C( =O)OR7, C( =O)NR8R9, C1-4 alkyl, wherein said C1-4 alkyl is optionally substituted with hydroxy, NR8R9 or a 5- or 6-membered heterocyclic ring, wherein said 5- or 6-membered heterocyclic ring is optionally substituted with C1-4 alkyl or C(=O)R7; or R5 and R6, together with the carbon atom to which they are attached, form =CH2 or a 5- or 6-membered heterocycloalkyl, wherein said heterocycloalkyl is optionally substituted with C1-4 alkyl; R7 is hydrogen or C1-4 alkyl; R8 and R9 are independently hydrogen or C1-4 alkyl, or R8 and R9, together with the nitrogen atom to which they are attached, form a 5- or 6-membered heterocyclic ring, or a pharmaceutically acceptable salt or solvate thereof. The invention relates further to intermediates for the preparation of said compounds, to said compounds for use in therapy, to pharmaceutical compositions comprising said compounds, to methods of treating or ameliorating pruritic dermal diseases or conditions with said compounds, and to the use of said compounds in the manufacture of medicaments.本发明涉及一种根据公式(A)的化合物,其中n为1或2;R1和R2独立为氢,C1-4烷基,C1-4卤代烷基,C1-4烷氧基,CD3或卤素;R3为氢,C(=O)OR7或C1-4烷基,可选地被羟基或NR8R9取代;R4为氢或氧代;R5和R6独立为氢,羟基,NR8R9,C(=O)R7,C(=O)OR7,C(=O)NR8R9,C1-4烷基,其中所述C1-4烷基可选地被羟基,NR8R9或5-或6-成员的杂环环取代,其中所述5-或6-成员的杂环环可选地被C1-4烷基或C(=O)R7取代;或R5和R6与它们所连接的碳原子一起形成=CH2或5-或6-成员的杂环烷基,其中所述杂环烷基可选地被C1-4烷基取代;R7为氢或C1-4烷基;R8和R9独立为氢或C1-4烷基,或R8和R9与它们所连接的氮原子一起形成一个5-或6-成员的杂环环,或其药用可接受的盐或溶剂化物。本发明进一步涉及用于制备所述化合物的中间体,所述化合物用于治疗,包含所述化合物的药物组合物,使用所述化合物治疗或改善瘙痒性皮肤病或状况的方法,以及所述化合物在药物制造中的用途。
-
Cobalt(ii)-catalyzed asymmetric hydrosilylation of simple ketones using dipyridylphosphine ligands in air作者:Feng Yu、Xi-Chang Zhang、Fei-Fei Wu、Ji-Ning Zhou、Wenjun Fang、Jing Wu、Albert S. C. ChanDOI:10.1039/c1ob05494e日期:——In the presence of PhSiH3 as the hydride donor, catalytic amounts of non-racemic dipyridylphosphine and an easy-to-handle cobalt salt Co(OAc)2·4H2O formed in situ an effective catalyst system for the asymmetric reduction of a diverse range of aryl alkyl ketones with moderate-to-excellent enantioselectivities (up to 96% ee). This approach tolerated the handling of both catalyst and reactants under air without special precautions.
-
Nickel(II)‐Dipyridylphosphine‐Catalyzed Enantioselective Hydrosilylation of Ketones in Air作者:Fei‐Fei Wu、Ji‐Ning Zhou、Qiang Fang、Yi‐Hu Hu、Shijun Li、Xi‐Chang Zhang、Albert S. C. Chan、Jing WuDOI:10.1002/asia.201200512日期:2012.11dipyridylphosphine ligand, as well as the stoichiometric hydride source PhSiH3, formed an effective catalyst system for the NiII‐catalyzed asymmetric hydrosilylation of a diverse range of electron‐deficient aryl alkyl ketones with enantioselectivities up to 90 % ee. The practical potential of the protocol was evinced by its good air‐stability.
-
Compounds and compositons for treating C1s-mediated diseases and conditions申请人:3-Dimensional Pharmaceuticals, Inc.公开号:US20020037915A1公开(公告)日:2002-03-28Disclosed is a method for treating the symptoms of an acute or chronic disorder mediated by the classical pathway of the complement cascade, comprising administering to a mammal in need of such treatment a therapeutically effective amount of a compound of Formula I 1 or a solvate, hydrate or pharmaceutically acceptable salt thereof; wherein R 1 , R 2 , R 3 , R 4 , X, Y and Z are defined in the specification.揭示了一种治疗急性或慢性疾病症状的方法,该疾病是由补体级联的经典途径介导的,包括向需要此类治疗的哺乳动物施用化合物I的治疗有效量或其溶剂化合物、水合物或药用可接受盐;其中规范中定义了R1、R2、R3、R4、X、Y和Z。
-
An Approach for the Synthesis of Pyrazolo[1,5-<i>a</i>]pyrimidines via Cu(II)-Catalyzed [3+3] Annulation of Saturated Ketones with Aminopyrazoles作者:Jian Ren、Shihua Ding、Xiaonian Li、Ran Bi、Qinshi ZhaoDOI:10.1021/acs.joc.1c01343日期:2021.9.17A one-step synthesis of diversely substituted pyrazolo[1,5-a]pyrimidines from saturated ketones and 3-aminopyrazoles is presented. This transformation involves the in situ formation of α,β-unsaturated ketones via a radical process, followed by [3+3] annulation with 3-aminopyrazoles in one pot. Mechanistic studies have shown that the dual C(sp3)–H bond functionalization of inactive ketones is required
表征谱图
-
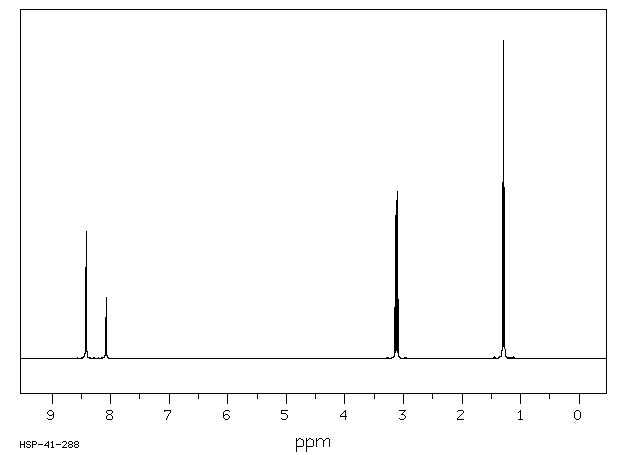
氢谱1HNMR
-
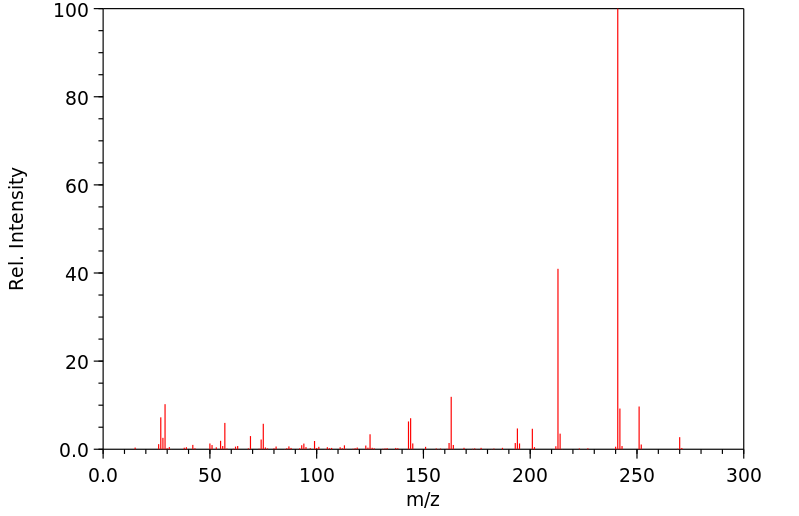
质谱MS
-
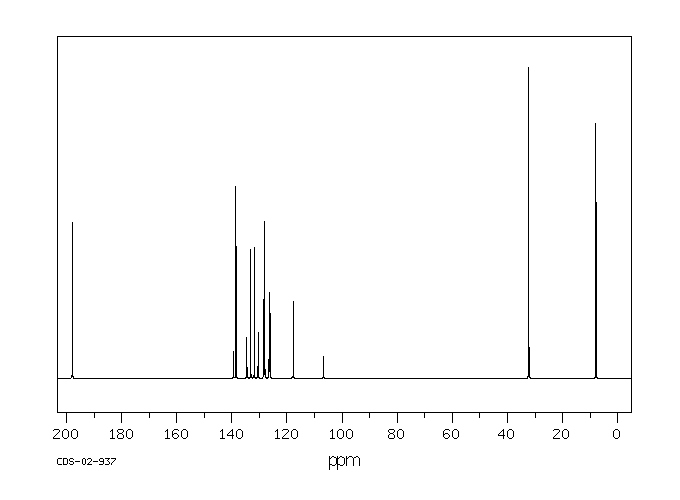
碳谱13CNMR
-
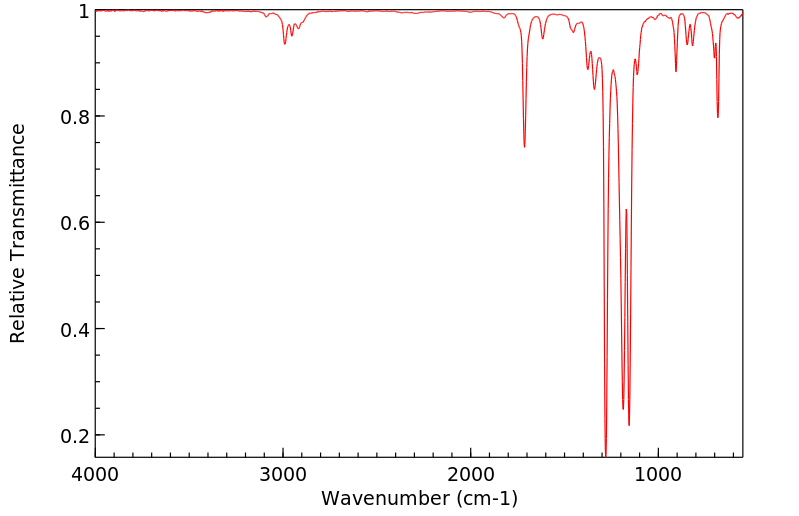
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷