3-chloro-2-norbornanone | 61914-03-2
中文名称
——
中文别名
——
英文名称
3-chloro-2-norbornanone
英文别名
3-chlorobicyclo[2.2.1]heptan-2-one
CAS
61914-03-2
化学式
C7H9ClO
mdl
MFCD00167574
分子量
144.601
InChiKey
PQRKEKMZLKKQOP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:9
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.857
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 降樟脑 Norbornan-2-on 497-38-1 C7H10O 110.156
反应信息
-
作为反应物:描述:3-chloro-2-norbornanone 在 sodium sulfide 作用下, 以 乙醇 、 水 为溶剂, 反应 5.0h, 以72%的产率得到Di(2-oxo-norbornan-3-yl)disulfid参考文献:名称:Martinetz, Dieter, Zeitschrift fur Chemie, 1985, vol. 25, # 4, p. 140摘要:DOI:
-
作为产物:描述:bicyclo[2.2.1]heptan-2-ol 在 N,N'-二甲基硫脲 、 N-氯代丁二酰亚胺 作用下, 以 二氯甲烷 为溶剂, 反应 1.0h, 以81%的产率得到endo-2-chloronorbornane参考文献:名称:硫脲介导的醇卤化反应。摘要:提出了在亚化学计量的硫脲添加剂的存在下加速的在温和条件下醇的卤化。硫脲的添加量决定了反应的途径,该途径可能与所需的卤化反应不同,在没有硫脲的情况下朝着醇的氧化,或者在使用过量的硫脲时朝着原料的回收。溴化和氯化对于伯醇,仲醇,叔醇和苄醇都非常有效,并且可以耐受各种官能团。详细的电子顺磁共振(EPR)研究,同位素标记和其他对照实验表明,基于自由基的机理。反应是在环境条件下进行的,使用的试剂普遍存在且价格便宜,具有广泛的应用范围,并且可以实现高度原子经济,DOI:10.1021/acs.joc.0c01431
文献信息
-
Enediolates of Carboxylic Acids in Synthesis: Synthesis of γ-Chloro-β-hydroxy Acids作者:Margarita Parra、Salvador Gil、María Kneeteman、Enrique SotocaDOI:10.1055/s-2002-19811日期:——Enediolates from carboxylic acids react readily with cyclic α-chloroketones to give the corresponding γ-chloro-β-alkoxycarboxylate intermediates depending on the ring size. Small ring ketones lead to γ-chloro-β-hydroxy acids in a highly stereoselective way, whereas medium ring ketones give a mixture of β,γ-epoxy acids and γ-lactones.
-
Reactions of halonorbornane and oxo-substituted derivatives with different anions by the electron transfer mechanism; redox catalysis in stabilized radicals作者:Jorge G. Uranga、Ana N. SantiagoDOI:10.1039/b9nj00503j日期:——3-chloronorbornan-2-one and 3-bromocamphor with Me3Sn−, Ph2P− or PhS− ions were studied by an SRN1 mechanism in liquid ammonia or DMSO. The results show that substrates having a carbonyl group facilitate electron transfer reactions, which are impeded in the absence of such a group. However, when the free radical formed is stabilized by conjugation, the coupling reaction decreases, causing a concomitant increase
-
Non-imidazole alkylamines as histamine H3-receptor ligands and their therapeutic applications申请人:——公开号:US20040220225A1公开(公告)日:2004-11-04Use of a compound of formula (A), wherein: 1 W is a residue which imparts antagonistic and/or agonistic activity at histamine H 3 -receptors when attached to an imidazole ring in 4(5) position; R 1 and R 2 may be identical or different and represent each independently a lower alkyl or cycloalkyl, or taken together with the nitrogen atom to which they are attached, a saturated nitrogen-containing ring (i) as defined, a non-aromatic unsaturated nitrogen-containing ring (ii) as defined, a morpholino group, or a N-substituted piperazino group as defined for preparing medicaments acting as antagonists and/or agonists at the H 3 -receptors of histamine.
-
Non-imidazole alkylamines as histamine H3- receptor ligands and their therapeutic applications申请人:Schwartz Jean-Charles公开号:US20060247223A1公开(公告)日:2006-11-02Use of a compound of formula (A), wherein: W is a residue which imparts antagonistic and/or agonistic activity at histamine H 3 -receptors when attached to an imidazole ring in 4(5) position; R 1 and R 2 may be identical or different and represent each independently a lower alkyl or cycloalkyl, or taken together with the nitrogen atom to which they are attached, a saturated nitrogen-containing ring (i) as defined, a non-aromatic unsaturated nitrogen-containing ring (ii) as defined, a morpholino group, or a N-substituted piperazino group as defined for preparing medicaments acting as antagonists and/or agonists at the H 3 -receptors of histamine.
-
NON-IMIDAZOLE ALKYLAMINES AS HISTAMINE H3-RECEPTOR LIGANDS AND THEIR THERAPEUTIC APPLICATIONS申请人:SCHWARTZ Jean-Charles公开号:US20110281844A1公开(公告)日:2011-11-17Use of a compound of formula (A), wherein: W is a residue which imparts antagonistic and/or agonistic activity at histamine H 3 -receptors when attached to an imidazole ring in 4(5) position; R 1 and R 2 may be identical or different and represent each independently a lower alkyl or cycloalkyl, or taken together with the nitrogen atom to which they are attached, a saturated nitrogen-containing ring (i) as defined, a non-aromatic unsaturated nitrogen-containing ring (ii) as defined, a morpholino group, or a N-substituted piperazino group as defined for preparing medicaments acting as antagonists and/or agonists at the H 3 -receptors of histamine.
表征谱图
-
氢谱1HNMR
-
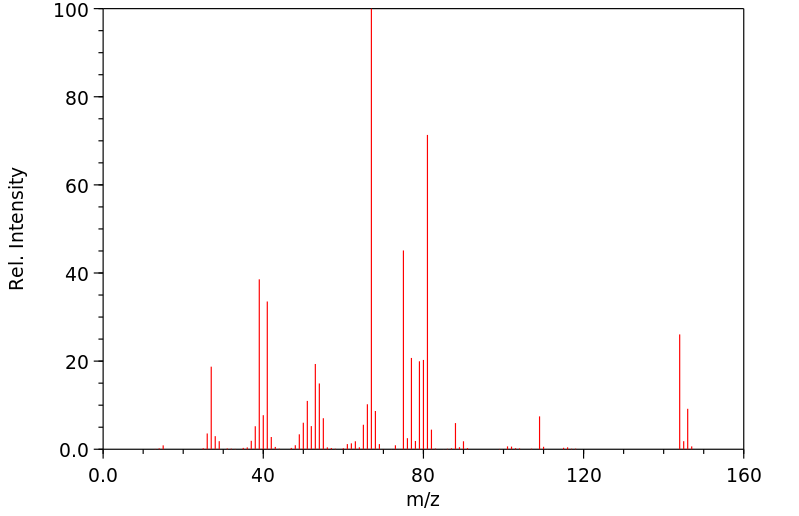
质谱MS
-
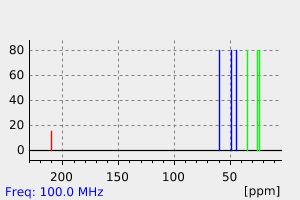
碳谱13CNMR
-
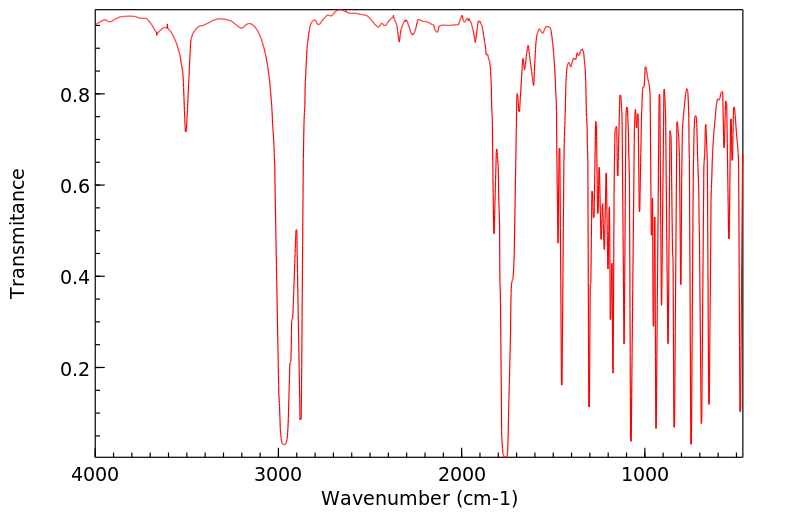
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β,6α,8α,10α,13α)-6-羟基-15-氧代黄-9(11),16-二烯-18-油酸
(3S,3aR,8aR)-3,8a-二羟基-5-异丙基-3,8-二甲基-2,3,3a,4,5,8a-六氢-1H-天青-6-酮
(2Z)-2-(羟甲基)丁-2-烯酸乙酯
(2S,4aR,6aR,7R,9S,10aS,10bR)-甲基9-(苯甲酰氧基)-2-(呋喃-3-基)-十二烷基-6a,10b-二甲基-4,10-dioxo-1H-苯并[f]异亚甲基-7-羧酸盐
(1aR,4E,7aS,8R,10aS,10bS)-8-[((二甲基氨基)甲基]-2,3,6,7,7a,8,10a,10b-八氢-1a,5-二甲基-氧杂壬酸[9,10]环癸[1,2-b]呋喃-9(1aH)-酮
(+)顺式,反式-脱落酸-d6
龙舌兰皂苷乙酯
龙脑香醇酮
龙脑烯醛
龙脑7-O-[Β-D-呋喃芹菜糖基-(1→6)]-Β-D-吡喃葡萄糖苷
龙牙楤木皂甙VII
龙吉甙元
齿孔醇
齐墩果醛
齐墩果酸苄酯
齐墩果酸甲酯
齐墩果酸溴乙酯
齐墩果酸二甲胺基乙酯
齐墩果酸乙酯
齐墩果酸3-O-alpha-L-吡喃鼠李糖基(1-3)-beta-D-吡喃木糖基(1-3)-alpha-L-吡喃鼠李糖基(1-2)-alpha-L-阿拉伯糖吡喃糖苷
齐墩果酸 beta-D-葡萄糖酯
齐墩果酸 beta-D-吡喃葡萄糖基酯
齐墩果酸 3-乙酸酯
齐墩果酸 3-O-beta-D-葡吡喃糖基 (1→2)-alpha-L-吡喃阿拉伯糖苷
齐墩果酸
齐墩果-12-烯-3b,6b-二醇
齐墩果-12-烯-3,24-二醇
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,11-二酮
齐墩果-12-烯-2α,3β,28-三醇
齐墩果-12-烯-29-酸,3,22-二羟基-11-羰基-,g-内酯,(3b,20b,22b)-
齐墩果-12-烯-28-酸,3-[(6-脱氧-4-O-b-D-吡喃木糖基-a-L-吡喃鼠李糖基)氧代]-,(3b)-(9CI)
齐墩果-12-烯-28-酸,3,7-二羰基-(9CI)
齐墩果-12-烯-28-酸,3,21,29-三羟基-,g-内酯,(3b,20b,21b)-(9CI)
鼠特灵
鼠尾草酸醌
鼠尾草酸
鼠尾草酚酮
鼠尾草苦内脂
黑蚁素
黑蔓醇酯B
黑蔓醇酯A
黑蔓酮酯D
黑海常春藤皂苷A1
黑檀醇
黑果茜草萜 B
黑五味子酸
黏黴酮
黏帚霉酸