5-二乙基胺基-2-戊醇 | 5412-69-1
中文名称
5-二乙基胺基-2-戊醇
中文别名
5-二乙氨基-2-戊醇
英文名称
5-diethylaminopentan-2-ol
英文别名
5-diethylamino-2-pentanol;5-(diethylamino)pentan-2-ol
CAS
5412-69-1
化学式
C9H21NO
mdl
——
分子量
159.272
InChiKey
OAQYRNDEOJQVBN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:284.8°C (rough estimate)
-
密度:0.86
-
稳定性/保质期:
常温常压下稳定,避免与强氧化剂接触。
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:11
-
可旋转键数:6
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:23.5
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险品标志:Xi
-
安全说明:S24/25
-
危险类别码:R36/37/38
-
海关编码:2922199090
SDS
| Name: | 5-Diethylamino-2-Pentanol 96% Material Safety Data Sheet |
| Synonym: | None known |
| CAS: | 5412-69-1 |
Synonym:None known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 5412-69-1 | 5-Diethylamino-2-Pentanol | 99 | 226-497-6 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
Causes eye irritation. May cause chemical conjunctivitis.
Skin:
Causes skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated.
Inhalation:
Causes respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. Can produce delayed pulmonary edema.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Use water spray to keep fire-exposed containers cool. Water may be ineffective.
Material is lighter than water and a fire may be spread by the use of water. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. Containers may explode when heated.
Extinguishing Media:
Water may be ineffective. Use agent most appropriate to extinguish fire. Cool containers with flooding quantities of water until well after fire is out. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions. Absorb spill using an absorbent, non-combustible material such as earth, sand, or vermiculite. Do not use combustible materials such as sawdust.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 5412-69-1: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: clear yellow
Odor: amine-like
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: .8660g/cm3
Molecular Formula: C9H21NO
Molecular Weight: 159.27
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 5412-69-1 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
5-Diethylamino-2-Pentanol - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 5412-69-1: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 5412-69-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 5412-69-1 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 4-hydroxy-valeric acid diethylamide 112146-12-0 C9H19NO2 173.255 5-二乙氨基-2-戊酮 5-diethylamino-2-pentanone 105-14-6 C9H19NO 157.256 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-氨基-5-二乙基氨基戊烷 5-diethylamino-2-pentylamine 140-80-7 C9H22N2 158.287 二乙基-(4-氯-戊基)-胺 diethyl-(4-chloro-pentyl)-amine 39749-10-5 C9H20ClN 177.717 —— diethyl-(4-bromo-pentyl)-amine 74204-01-6 C9H20BrN 222.168
反应信息
-
作为反应物:描述:参考文献:名称:合成关键中间体 novoldiamine 和 hydroxynovaldiamine 的可扩展工艺及其在氯喹、羟氯喹和 mepacrine 合成中的应用摘要:摘要 一种高效且可扩展的合成酚醛二胺和羟基酚醛二胺的工艺是由乙酰丙酸完成的,乙酰丙酸很容易从天然原料中获得。这些二胺被用作合成氯喹、羟氯喹和甲巴林的关键中间体。该过程涉及的关键步骤是乙酰丙酸与仲胺在1, 1'-羰基二咪唑的辅助下偶联和肟-酰胺官能团的一锅还原得到相应的二胺。DOI:10.1080/00397911.2022.2061358
-
作为产物:描述:参考文献:名称:Tsuda; Yoshida; Yamashina, Yakugaku Zasshi/Journal of the Pharmaceutical Society of Japan, 1946, vol. 66, p. 59摘要:DOI:
文献信息
-
[EN] PRODRUG BIPYRIDYLAMINOPYRIDINES AS SYK INHIBITORS<br/>[FR] PROMÉDICAMENT BIPYRIDYLAMINOPYRIDINES EN TANT QU'INHIBITEURS DE SYK申请人:MERCK SHARP & DOHME公开号:WO2014074421A1公开(公告)日:2014-05-15The present invention provides compounds of Formula (I), which are prodrugs of trans-4-[(1R)-(6-[4-(difluoromethyl)pyridin-2-yl]amino}-4-methyl-2,3'-bipyridin-6'-yl)-1-hydroxyethyl]cyclohexanecarboxylic acid, a potent inhibitor of Syk. The compounds are useful in the treatment and prevention of diseases mediated by the enzyme, such as asthma, COPD, rheumatoid arthritis and cancer.
-
Detritylation Procedure under Non-Acidic Conditions: Naphthalene Catalysed Reductive Cleavage of Trityl Ethers作者:Miguel Yus、Cherif Behloul、David GuijarroDOI:10.1055/s-2003-41057日期:——benzylic trityl ethers 1 with lithium powder and a catalytic amount of naphthalene led to reductive cleavage of the trityl-oxygen bond, affording the corresponding alcohols 2 in good to excellent yields under very mild reaction conditions. The detritylation process could successfully be extended to several hydroxy, alkoxy and amino functionalised trityl ethers. This methodology represents a new and efficient
-
[EN] 6-HYDROXYBENZOFURANYL- AND 6-ALKOXYBENZOFURANYL-SUBSTITUTED IMIDAZOPYRIDAZINES<br/>[FR] IMIDAZOPYRIDAZINES 6-HYDROXYBENZOFURANYL- ET 6-ALCOXYBENZOFURANYL-SUBSTITUÉES申请人:BAYER PHARMA AG公开号:WO2016102427A1公开(公告)日:2016-06-30The present invention relates to 6-hydroxybenzofuranyl- and 6-alkoxybenzofuranyl- substituted imidazopyridazine compounds of general formula (I) in which R1 and R2 are as defined in the claims, to methods of preparing said compounds, to intermediate compounds useful for preparing said compounds, to pharmaceutical compositions and combinations comprising said compounds and to the use of said compounds for manufacturing a pharmaceutical composition for the treatment or prophylaxis of a disease, in particular of a hyper-proliferative and/or angiogenesis disorder, as a sole agent or in combination with other active ingredients.
-
METHOD FOR THE CONTINUOUS PRODUCTION OF AN AMINE USING AN ALUMINUM-COPPER CATALYST申请人:Ernst Martin公开号:US20110172430A1公开(公告)日:2011-07-14Process for the continuous preparation of an amine by reaction of a primary or secondary alcohol, aldehyde and/or ketone with hydrogen and a nitrogen compound selected from the group consisting of ammonia, primary and secondary amines at a temperature in the range from 60 to 300° C. in the presence of a catalyst comprising copper oxide and aluminum oxide, wherein the reaction takes place in the gas phase and the catalytically active composition of the catalyst before reduction with hydrogen comprises PS from 20 to 75% by weight of aluminum oxide (Al 2 O 3 ), from 20 to 75% by weight of oxygen-comprising compounds of copper, calculated as CuO, from 0 to 2% by weight of oxygen-comprising compounds of sodium, calculated as Na 2 O, and less than 5% by weight of oxygen-comprising compounds of nickel, calculated as NiO, and the shaped catalyst body has a pellet shape having a diameter in the range from 1 to 4 mm and a height in the range from 1 to 4 mm.
-
Synthesis and spasmolytic activity of aminoalkyl 2-substituted-2-(1,2-benzisoxazol-3-yl)acetates.作者:SHUNSUKE NARUTO、SHOZO UEDA、TOYOKICHI YOSHIDA、HIROYUKI MIZUTA、KATSUYOSHI KAWASHIMA、TOSHIAKI KADOKAWADOI:10.1248/cpb.35.2095日期:——Several aminoalkyl esters of 2- (1, 2-benzisoxazol-3-yl) -2-cyclohexylacetic acid, 1- (1, 2-benzisoxazol-3-yl) -1-cyclopentanecarboxylic acid, and 1- (1, 2-benzisoxazol-3-yl) -1-cyclohexanecarboxylic acid and their quaternary ammonium salts were synthesized. The anticholinergic (anti-Ach) and musculotropic (anti-KCl) activities of these compounds were examined. Among them, 3- (N, N-diethylamino) -propyl 2- (1, 2-benzisoxazol-3-yl) -2-cyclohexylacetate (8b) showed potent anti-Ach and anti-KCl activities.
表征谱图
-
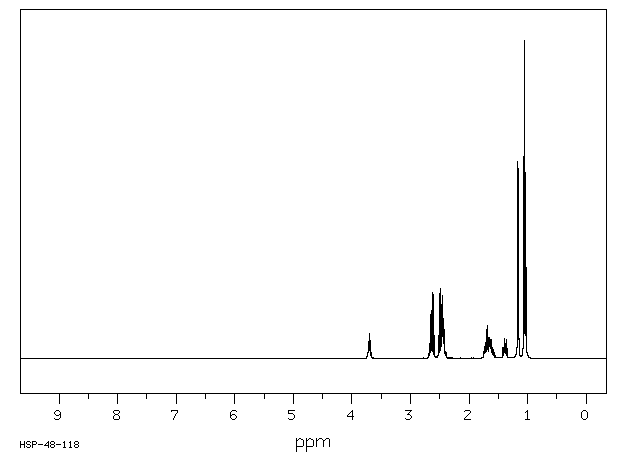
氢谱1HNMR
-
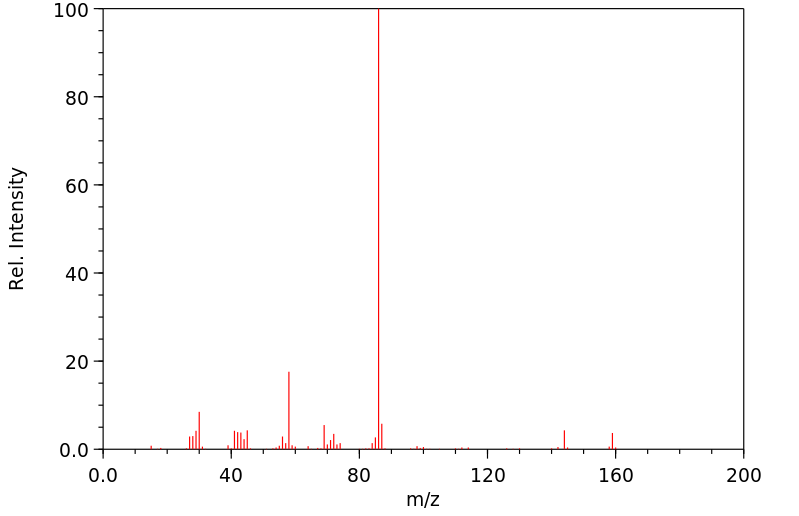
质谱MS
-
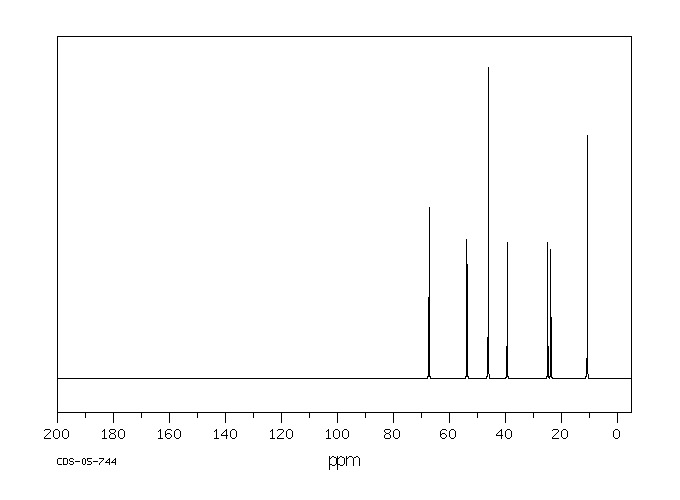
碳谱13CNMR
-
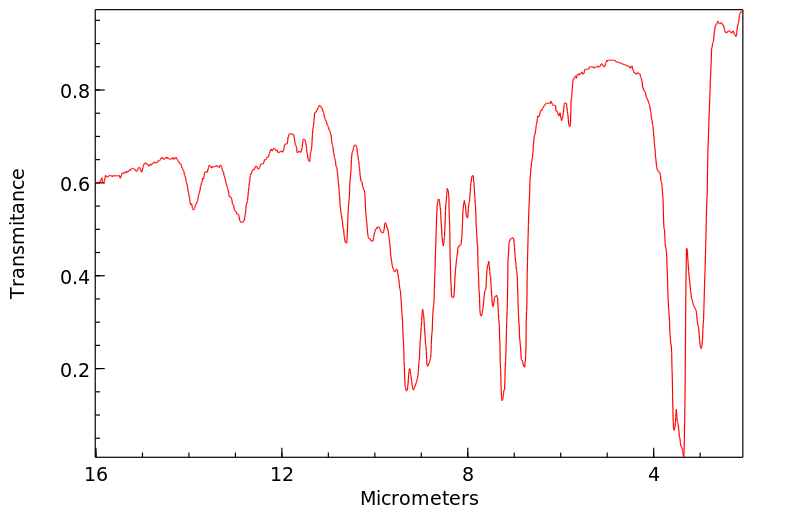
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷