3,3-二甲基-1-己烯 | 3404-77-1
中文名称
3,3-二甲基-1-己烯
中文别名
3,3-二甲基-1-己烷
英文名称
3,3-dimethyl-1-hexene
英文别名
3,3-dimethylhex-1-ene;3,3-Dimethyl-hex-1-en;3,3-Dimethyl-hexen
CAS
3404-77-1
化学式
C8H16
mdl
MFCD00048727
分子量
112.215
InChiKey
RXYYKIMRVXDSFR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-103.01°C (estimate)
-
沸点:104 °C
-
密度:0.72
-
保留指数:718
-
稳定性/保质期:
在常温常压下保持稳定。
计算性质
-
辛醇/水分配系数(LogP):3.8
-
重原子数:8
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险等级:3
-
海关编码:2901299090
-
危险品运输编号:UN 3295
-
储存条件:常温、避光、通风干燥处,密封保存。
SDS
3,3-二甲基-1-己烯 修改号码:2
模块 1. 化学品
产品名称: 3,3-Dimethyl-1-hexene
修改号码: 2
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第2级
健康危害
吸入性危害物质 第1级
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 高度易燃液体和蒸气
若吞咽并进入呼吸道可能致命
防范说明
[预防] 远离热源/火花/明火/热表面。禁烟。
保持容器密闭。
使用防爆的电气/通风/照明设备。采取预防措施以防静电和火花引起的着火。
穿戴防护手套/护目镜/防护面具。
[急救措施] 食入:立即呼叫解毒中心/医生。切勿催吐。
皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
[储存] 存放于通风良好处。保持凉爽。
存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 3,3-二甲基-1-己烯
百分比: >98.0%(GC)
CAS编码: 3404-77-1
3,3-二甲基-1-己烯 修改号码:2
模块 3. 成分/组成信息
分子式: C8H16
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 立即呼叫解毒中心/医生。漱口。切勿引吐。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:喷水,保持容器冷却。如果安全,消除一切火源。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(自携式呼吸器)。远离溢出物/泄露处并处在上风处。确保
紧急措施: 足够通风。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 回收到密闭容器前用干砂或惰性吸收剂吸收泄漏物。一旦大量泄漏,筑堤控制。附着
物或收集物应该根据相关法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离热源/火花/明火
/热表面。禁烟。采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 如果可能,使用封闭系统。如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 半面罩或全面罩呼吸器,自携式呼吸器(SCBA),供气呼吸器等。依据当地和政府法
规,使用通过政府标准的呼吸器。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
3,3-二甲基-1-己烯 修改号码:2
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-几乎无色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 104 °C
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.72
溶解度: 无资料
模块 10. 稳定性和反应性
稳定性: 一般情况下稳定。
反应性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧,焚烧时需要特别注
意该物质是高度可燃的。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第3类 易燃液体 。
UN编号: 3295
3,3-二甲基-1-己烯 修改号码:2
模块 14. 运输信息
正式运输名称: 碳氢化合物, 液体, 不另作详细说明
包装等级: II
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布): 针对危险化学品的安全使用、生产、储存、运输、装
卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 3,3-Dimethyl-1-hexene
修改号码: 2
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第2级
健康危害
吸入性危害物质 第1级
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 高度易燃液体和蒸气
若吞咽并进入呼吸道可能致命
防范说明
[预防] 远离热源/火花/明火/热表面。禁烟。
保持容器密闭。
使用防爆的电气/通风/照明设备。采取预防措施以防静电和火花引起的着火。
穿戴防护手套/护目镜/防护面具。
[急救措施] 食入:立即呼叫解毒中心/医生。切勿催吐。
皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
[储存] 存放于通风良好处。保持凉爽。
存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 3,3-二甲基-1-己烯
百分比: >98.0%(GC)
CAS编码: 3404-77-1
3,3-二甲基-1-己烯 修改号码:2
模块 3. 成分/组成信息
分子式: C8H16
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 立即呼叫解毒中心/医生。漱口。切勿引吐。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:喷水,保持容器冷却。如果安全,消除一切火源。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(自携式呼吸器)。远离溢出物/泄露处并处在上风处。确保
紧急措施: 足够通风。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 回收到密闭容器前用干砂或惰性吸收剂吸收泄漏物。一旦大量泄漏,筑堤控制。附着
物或收集物应该根据相关法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离热源/火花/明火
/热表面。禁烟。采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 如果可能,使用封闭系统。如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 半面罩或全面罩呼吸器,自携式呼吸器(SCBA),供气呼吸器等。依据当地和政府法
规,使用通过政府标准的呼吸器。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
3,3-二甲基-1-己烯 修改号码:2
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-几乎无色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 104 °C
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.72
溶解度: 无资料
模块 10. 稳定性和反应性
稳定性: 一般情况下稳定。
反应性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧,焚烧时需要特别注
意该物质是高度可燃的。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第3类 易燃液体 。
UN编号: 3295
3,3-二甲基-1-己烯 修改号码:2
模块 14. 运输信息
正式运输名称: 碳氢化合物, 液体, 不另作详细说明
包装等级: II
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布): 针对危险化学品的安全使用、生产、储存、运输、装
卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
反应信息
-
作为反应物:描述:参考文献:名称:Bolschuchin; Egorow, Zhurnal Obshchei Khimii, 1957, vol. 27, p. 933,934;engl.Ausg.S.1014,1015摘要:DOI:
-
作为产物:描述:o-toluenesulfonate of 4,4-dimethyl-hex-5-en-1-ol 在 lithium aluminium tetrahydride 作用下, 以 乙醚 为溶剂, 反应 20.0h, 以39%的产率得到3,3-二甲基-1-己烯参考文献:名称:Beckwith, Athelstan L. J.; Easton, Christopher J.; Lawrence, Tony, Australian Journal of Chemistry, 1983, vol. 36, # 3, p. 545 - 556摘要:DOI:
文献信息
-
Cross-metathesis reaction of functionalized and substituted olefins using group 8 transition metal carbene complexes as metathesis catalysts申请人:CALIFORNIA INSTITUTE OF TECHNOLOGY公开号:US09403854B2公开(公告)日:2016-08-02The invention pertains to the use of Group 8 transition metal carbene complexes as catalysts for olefin cross-metathesis reactions. In particular, ruthenium and osmium alkylidene complexes substituted with an N-heterocyclic carbene ligand are used to catalyze cross-metathesis reactions to provide a variety of substituted and functionalized olefins, including phosphonate-substituted olefins, directly halogenated olefins, 1,1,2-trisubstituted olefins, and quaternary allylic olefins. The invention further provides a method for creating functional diversity using the aforementioned complexes to catalyze cross-metathesis reactions of a first olefinic reactant, which may or may not be substituted with a functional group, with each of a plurality of different olefinic reactants, which may or may not be substituted with functional groups, to give a plurality of structurally distinct olefinic products. The methodology of the invention is also useful in facilitating the stereoselective synthesis of 1,2-disubstituted olefins in the cis configuration.
-
A General Model for Selectivity in Olefin Cross Metathesis作者:Arnab K. Chatterjee、Tae-Lim Choi、Daniel P. Sanders、Robert H. GrubbsDOI:10.1021/ja0214882日期:2003.9.1When an olefin of high reactivity is reacted with an olefin of lower reactivity (sterically bulky, electron-deficient, etc.), selective cross metathesis can be achieved using feedstock stoichiometries as low as 1:1. By employing a metathesis catalyst with the appropriate activity, selective cross metathesis reactions can be achieved with a wide variety of electron-rich, electron-deficient, and sterically近年来,烯烃交叉复分解(CM)已成为有机化学中一种强大而方便的合成技术;然而,作为一种通用的合成方法,CM 受到产品选择性和立体选择性缺乏可预测性的限制。对几类烯烃(包括取代和官能化的苯乙烯、仲烯丙醇、叔烯丙醇和具有 α-季铵盐中心的烯烃)进行烯烃交叉复分解的研究,得出了一个通用模型,可用于预测交叉反应中的产物选择性和立体选择性。复分解。作为 CM 中烯烃反应性的一般排名,烯烃可以根据它们通过交叉复分解进行均二聚化的相对能力和它们的同二聚体对二级复分解反应的敏感性进行分类。当高反应性烯烃与低反应性烯烃(空间体积大、缺电子等)反应时,可以使用低至 1:1 的原料化学计量实现选择性交叉复分解。通过使用具有适当活性的复分解催化剂,可以与多种富电子、缺电子和空间大的烯烃实现选择性交叉复分解反应。该模型的应用允许预测和开发选择性交叉复分解反应,最终形成前所未有的三组分分子间交叉复分解反应。通过使用具
-
Rhodium-Catalyzed Cyclopropanation of Alkenes with Dimethyl Diazomalonate作者:Francisco González-Bobes、Michaël D. B. Fenster、Susanne Kiau、Laxma Kolla、Sergei Kolotuchin、Maxime SoumeillantDOI:10.1002/adsc.200800027日期:2008.4.7α′-tetramethyl-1,3-benzenedipropanoate] to catalyze the cyclopropanation of a wide range of alkenes with malonate-derived carbenoids under mild reaction conditions is reported in this communication. The experimental protocol is remarkably simple, uses readily accessible and stable dimethyl diazomalonate with very low catalyst loading. More importantly, the alkene is employed as a limiting reagent.
-
Co(II) Schiff base complex decorated on polysalicylaldehyde as an efficient, selective, heterogeneous and reusable catalyst for epoxidation of olefins in mild and self-coreductant conditions作者:Milad Kazemnejadi、Alireza Shakeri、Mahsa Nikookar、Mohammad Mohammadi、Mohsen EsmaeilpourDOI:10.1007/s11164-017-3027-z日期:2017.12new Co(II)-Schiff base complex was decorated on a polysalicylaldehyde (PSA) framework and used as a selective and efficient catalyst for the epoxidation of alkenes in the presence of O2 as a green source of oxygen without aco-reductant. The catalyst was characterized step by step by FTIR, UV–Vis, 1H NMR, TGA, CHN, XPS and EDX analyses. Loading an amount of Co ions in the catalyst as well as its leaching摘要 在聚水杨醛(PSA)骨架上装饰了一种新的Co(II)-席夫碱配合物,并在O 2作为绿色氧源而不存在助还原剂的情况下,用作烯烃环氧化的选择性和高效催化剂。通过FTIR,UV-Vis,1逐步表征催化剂1 H NMR,TGA,CHN,XPS和EDX分析。用ICP-OES仪器研究了催化剂中Co离子的负载量及其浸出量。该催化剂以温和,廉价和有效的方案表现出对多种烯烃优异的活性。同样,可以简单地从反应混合物中回收催化剂,并重复使用几次,而没有任何明显的活性损失。通过反应时间筛选反应参数,包括温度,氧气流量,催化剂量和溶剂。进行了包括XPS光谱学在内的催化研究和一些空白实验,以初步了解反应机理。另外,将新型催化剂的反应性评价为周转频率。 图形概要 已经开发了一种新的有效方案,用于在分子氧的作用下,在轻度和自共轭条件下,使用多水杨醛的多相可循环利用的Co(II)-席夫碱配合物对烯烃进行选择性环氧化。
-
Rhodium-catalyzed selective C–H functionalization of NNN tridentate chelating compounds via a rollover pathway作者:Seung Youn Hong、Jaesung Kwak、Sukbok ChangDOI:10.1039/c5cc09960a日期:——Reported herein is the first example of a Rh(NHC)-catalyzed selective bis C-H alkylation of NNN tridentate chelating compounds in reaction with alkenes. The observed excellent site-selectivity can readily be explained...本文报道的是Rh(NHC)催化的NNN三齿螯合化合物与烯烃反应的选择性双CH烷基化的第一个例子。观察到的出色的位点选择性可以很容易地解释。
表征谱图
-
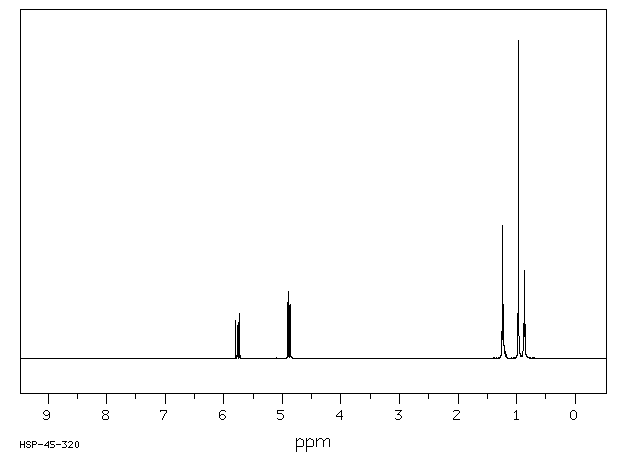
氢谱1HNMR
-
质谱MS
-
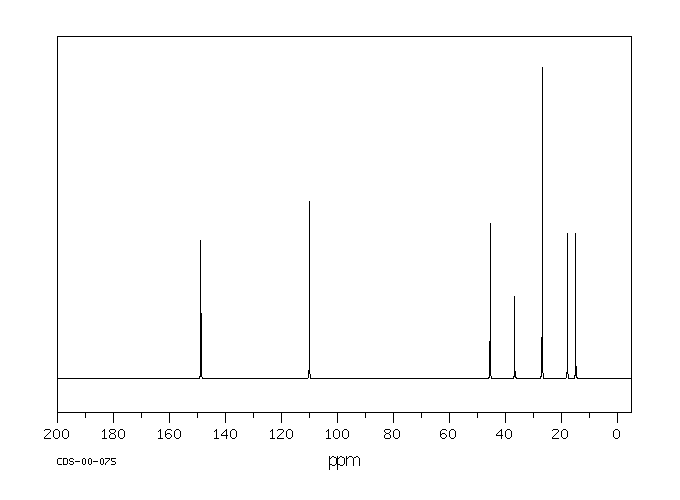
碳谱13CNMR
-
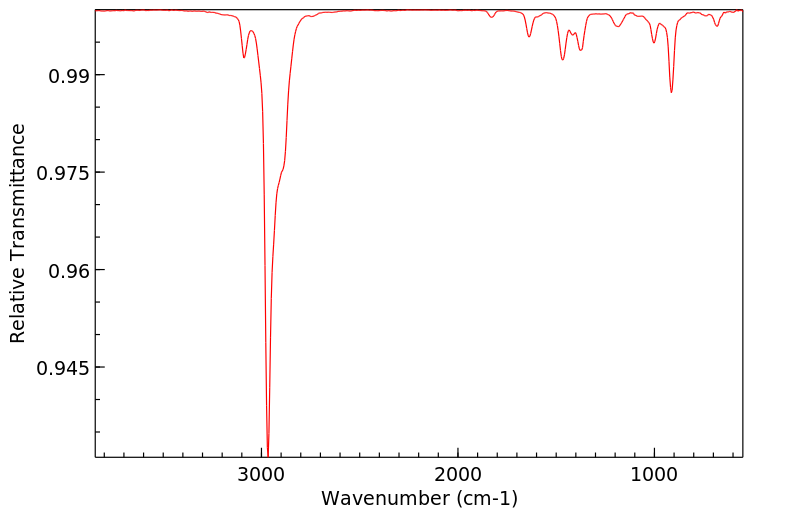
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-