6-硝基苯酞 | 610-93-5
中文名称
6-硝基苯酞
中文别名
6-硝基四氯苯酞;6-硝基-1,3-二氢异苯并呋喃-1-酮;6-硝苯酞酯
英文名称
6-nitrophthalide
英文别名
6-nitroisobenzofuran-1(3H)-one;6-nitro-3H-isobenzofuran-1-one;6-nitro-3H-2-benzofuran-1-one
CAS
610-93-5
化学式
C8H5NO4
mdl
MFCD00033529
分子量
179.132
InChiKey
RNWGZXAHUPFXLL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:140-145 °C(lit.)
-
沸点:311.63°C (rough estimate)
-
密度:1.4908 (rough estimate)
-
稳定性/保质期:
性质与稳定性: 在常温常压下,该物质不会分解。
计算性质
-
辛醇/水分配系数(LogP):0.6
-
重原子数:13
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.125
-
拓扑面积:72.1
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
安全说明:S24/25
-
危险类别码:R20/21/22,R36/37/38,R20/21/2
-
WGK Germany:3
-
海关编码:2932209090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:贮存: 将密器密封后,储存在密封的主容器中,并放置在阴凉、干燥的地方。
SDS
| Name: | 6-Nitrophthalide 97% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 610-93-5 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 610-93-5 | 6-Nitrophthalide | 97% | unlisted |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Not available.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract.
Inhalation:
May cause respiratory tract irritation.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 610-93-5: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: Not available.
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 140 - 145 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula:
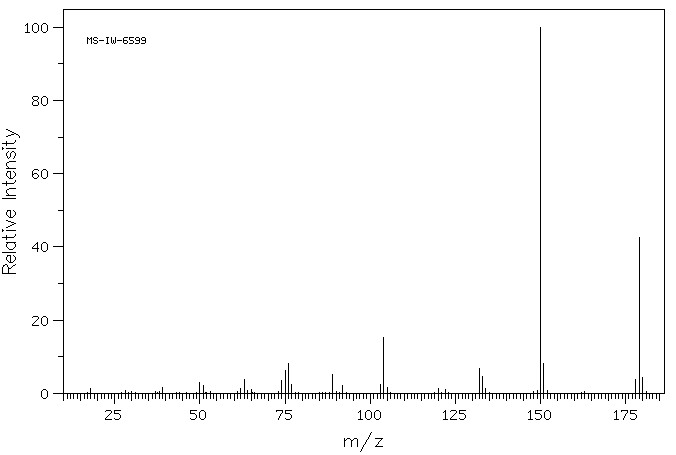
Molecular Weight: 179.13
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, oxides of nitrogen, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 610-93-5 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
6-Nitrophthalide - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 610-93-5: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 610-93-5 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 610-93-5 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-硝基邻苯二甲酸酐 4-nitro-phthalic anhydride 5466-84-2 C8H3NO5 193.116 2-甲基-5-硝基苯甲酸 5-nitro-o-toluic acid 1975-52-6 C8H7NO4 181.148 (4-硝基-1,2-亚苯基)二甲醇 (4-nitro-1,2-phenylene)dimethanol 22162-19-2 C8H9NO4 183.164 2-溴-5-硝基苯甲酸 2-bromo-5-nitrobenzoic acid 943-14-6 C7H4BrNO4 246.017 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3-hydroxy-6-nitroisobenzofuran-1(3H)-one 77619-93-3 C8H5NO5 195.131 —— 2-Hydroxymethyl-5-nitro-benzoesaeure 89942-82-5 C8H7NO5 197.147 2-甲酰基-5-硝基苯甲酸甲酯 methyl 2-formyl-5-nitrobenzoate 142314-69-0 C9H7NO5 209.158 —— 3-bromo-6-nitroisobenzofuran-1(3H)-one 89891-73-6 C8H4BrNO4 258.028 4-硝基邻苯二甲酸 4-nitrophthalic acid 610-27-5 C8H5NO6 211.131 2-甲酰基-5-硝基-苯甲酸 2-formyl-5-nitrobenzoic acid 7464-91-7 C8H5NO5 195.131 —— methyl 2-(chloromethyl)-5-nitrobenzoate 90537-42-1 C9H8ClNO4 229.62 6-氨基四氯苯酞 6-aminophthalide 57319-65-0 C8H7NO2 149.149 —— (R)-3-allyl-6-nitroisobenzofuran-1(3H)-one 1387557-72-3 C11H9NO4 219.197 —— 6-methylaminophthalide 874478-15-6 C9H9NO2 163.176 —— 3-benzylidene-6-nitro-3H-isobenzofuran-1-one 15298-16-5 C15H9NO4 267.241 —— 6-Dimethylamino-1(3H)-isobenzofuranon 65399-10-2 C10H11NO2 177.203 (4-硝基-1,2-亚苯基)二甲醇 (4-nitro-1,2-phenylene)dimethanol 22162-19-2 C8H9NO4 183.164 N-(3-氧代-1H-2-苯并呋喃-5-基)乙酰胺 6-acetylaminophthalide 21626-90-4 C10H9NO3 191.186 —— 3-(4-dimethylamino-benzylidene)-6-nitro-3H-isobenzofuran-1-one 40392-22-1 C17H14N2O4 310.309 —— 6-Benzylaminophthalide 1424363-45-0 C15H13NO2 239.274 —— 3-<4-Methoxy-benzyliden>-6-nitro-phthalid 40392-21-0 C16H11NO5 297.267 —— 3-(p-Methoxybenzyliden)-6-nitro-phthalid 40392-21-0 C16H11NO5 297.267 —— 6-amino-7-nitrophthalide 146767-10-4 C8H6N2O4 194.147 —— 6,7-diaminophthalide 146767-11-5 C8H8N2O2 164.164 —— 2-hydroxymethyl-5-nitro-N,N-dimethylbenzamide —— C10H12N2O4 224.216 —— 2-<1,3-dithian-2-yl>-5-nitrobenzoic acid 98015-13-5 C11H11NO4S2 285.345 —— 6-benzoylamino-phthalide 873395-19-8 C15H11NO3 253.257 —— 3-<3,4-Methylendioxy-benzyliden>-6-nitro-phthalid 57319-64-9 C16H9NO6 311.251 —— 6-acetylamino-7-nitrophthalide 146767-09-1 C10H8N2O5 236.184 —— 6-Amino-3-benzyliden-phthalid 15298-18-7 C15H11NO2 237.258 - 1
- 2
- 3
反应信息
-
作为反应物:参考文献:名称:铁和钯 (II) 酞菁作为还原硝基芳烃的可回收催化剂摘要:铁(II)和钯(II)酞菁已被确定为可回收的多相催化剂,用于使用二苯基硅烷/硼氢化钠作为乙醇中的氢源将芳香硝基化合物还原为相应的胺。各种可还原的官能团,如乙酰基、酯、氰基、酰胺、磺酰胺和羧酸等均具有良好的耐受性,并且该方法适用于克级规模。机理研究表明,硝基的还原通过直接(亚硝基)途径进行,可能铁或钯酞菁会激活硝基进行还原。FePc 和 PdPc 还催化二苯基硅烷/硼氢化钠和乙醇的组合产生氢气。图形摘要铁和钯(II)酞菁已被确立为一种有效的可回收催化体系,用于用绿色溶剂体系还原硝基芳烃。各种硝基取代的芳烃和杂芳烃已成功还原为相应的胺,产率非常好。本方法也已有效地适用于克级反应。DOI:10.1007/s10562-014-1269-6
-
作为产物:描述:参考文献:名称:作为正电子发射断层扫描和光学成像双峰标签潜在前体的邻碘碘苄醇‒BODIPY结构的合成摘要:为了使新型潜在示踪剂从实验台到床头的快速发展,正电子发射断层扫描(PET)和光学成像(OI)的多峰示踪剂已成为一种非常有前途的工具。的确,它们结合了使用光学技术进行体外/体内临床前研究的简便性,以及使用其放射性版本的PET成像所提供的各种临床可能性。在这种情况下,已经研究了新的标签的制备,该标签可通过荧光成像检测并且潜在地适用于相应的前体的最后一步11 C-标记后的PET成像。通过链接o探索各种设计和综合-碘苄基醇和四甲基-BODIPY部分一起。其中,最有希望的结构是由市售廉价的起始原料通过五个步骤以30%的产率生产的。DOI:10.1016/j.tet.2019.130765
-
作为试剂:描述:苯酞 、 potassium nitrate 在 水 、 6-硝基苯酞 作用下, 以 硫酸 为溶剂, 反应 16.0h, 以This resulted in 136 g (crude) of 6-nitroisobenzofuran-1(3H)-one as a white solid的产率得到6-硝基苯酞参考文献:名称:Cyclohexyl-azetidinyl antagonists of CCR2摘要:本发明涉及公式I的化合物,其中:R1、R2、R4、J、Q和A如规范中所定义。该发明还涉及一种预防、治疗或改善综合症、疾病或疾患的方法,其中所述综合症、疾病或疾患是2型糖尿病、肥胖症和哮喘。该发明还涉及通过给哺乳动物施用公式I中至少一种化合物的治疗有效量来抑制CCR2活性的方法。公开号:US09062048B2
文献信息
-
Compositions for Treatment of Cystic Fibrosis and Other Chronic Diseases申请人:Vertex Pharmaceuticals Incorporated公开号:US20150231142A1公开(公告)日:2015-08-20The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
Commercially Available CuO Catalyzed Hydrogenation of Nitroarenes Using Ammonia Borane as a Hydrogen Source作者:Jialei Du、Jie Chen、Hehuan Xia、Yiwei Zhao、Fang Wang、Hong Liu、Weijia Zhou、Bin WangDOI:10.1002/cctc.201902391日期:2020.5.7dehydrogenation and nitroarenes hydrogenation has been reported as a novel strategy for the preparation of aromatic amines. However, the practical application of this strategy is subjected to the high‐cost and tedious preparation of supported noble metal nanocatalysts. The commercially available CuO powder is herein demonstrated to be a robust catalyst for hydrogenation of nitroarenes using ammonia borane
-
COMPOSITIONS FOR TREATMENT OF CYSTIC FIBROSIS AND OTHER CHRONIC DISEASES申请人:Van Goor Fredrick F.公开号:US20110098311A1公开(公告)日:2011-04-28The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
[EN] CYCLOHEXYL-AZETIDINYL ANTAGONISTS OF CCR2<br/>[FR] ANTAGONISTES DU CCR2 À BASE DE CYCLOHEXYL-AZÉTIDINYLE申请人:JANSSEN PHARMACEUTICA NV公开号:WO2011159854A1公开(公告)日:2011-12-22The present invention comprises compounds of Formula (I). Wherein: R1, R2, R4, J, Q, and A are as defined in the specification. The invention also comprises a method of preventing, treating or ameliorating a syndrome, disorder or disease, wherein said syndrome, disorder or disease is type II diabetes, obesity and asthma. The invention also comprises a method of inhibiting CCR2 activity in a mammal by administration of a therapeutically effective amount of at least one compound of Formula (I).本发明包括公式(I)的化合物。其中:R1、R2、R4、J、Q和A如说明书所述定义。本发明还包括预防、治疗或改善综合征、障碍或疾病的方法,其中所述综合征、障碍或疾病为2型糖尿病、肥胖和哮喘。本发明还包括通过管理治疗有效量的至少一种公式(I)化合物来抑制哺乳动物中的CCR2活性的方法。
-
SULFONAMIDE DERIVATIVE AND MEDICINAL USE THEREOF申请人:AJINOMOTO CO., LTD.公开号:US20150051395A1公开(公告)日:2015-02-19Provided are sulfonamide derivatives of a specific chemical structure in which a sulfonamide group having, as a substituent, a phenyl group or a heterocyclic group having a hetero atom(s) as a constituent element(s) is present at its terminal, and pharmaceutically acceptable salts thereof. These compounds are novel compounds having excellent α4 integrin-inhibitory action.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
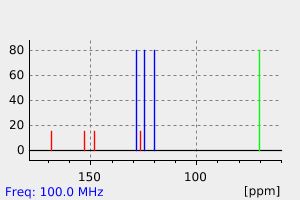
碳谱13CNMR
-
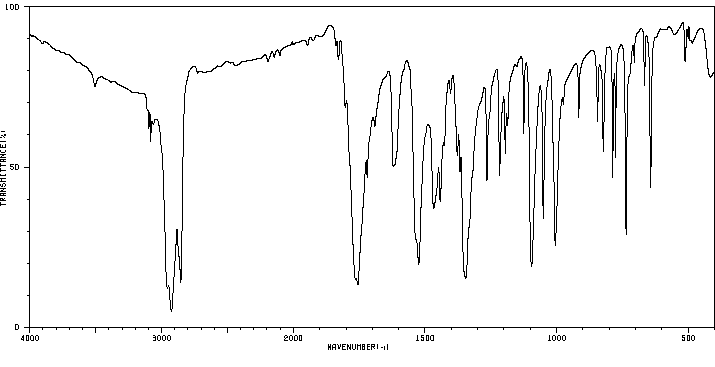
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
麦考酚酸羧丁氧基醚
麦考酚酸-d3
霉酚酸钠
霉酚酸酯杂质3
霉酚酸酯杂质1
霉酚酸酯EP杂质H(霉酚酸酯-USP相关化合物B)
霉酚酸甲酯
霉酚酸环丙烷类似物
霉酚酸吗啉乙酯
霉酚酸内酯
霉酚酸-(全碳-13)
霉酚酸
阿夫洛尔
袂康宁-d3
苯酞
艾司西酞普兰杂质
艾司西酞普兰杂质
盐酸氮淖斯汀杂质SM3-I5
盐酸氮卓斯汀杂质E
正丁亚基邻苯二甲酰胺
托格列净一水合物(1:1)
托格列净
异苯并呋喃,1-丁基-1,3-二氢-
异苯并呋喃,1-[(3-氯苯基)亚甲基]-1,3-二氢-,(Z)-
川芎内酯E
川芎内酯 C
四氯苯酞
呋吡菌胺
亚苄基酞
丁苯酞标准品028
{3-[5-溴-1-苯基-3,3-二甲基-1,3-二氢-异苯并呋喃-1-基]-丙基}-二甲基-胺
m-袂康宁
Z-亚丁基苯酞
O-甲基吗替麦考酚酯
N-苯基邻苯二甲酰亚胺高氯酸盐
N-环己基邻苯二甲酰亚胺高氯酸盐
N-(3-氧代-1H-2-苯并呋喃-5-基)乙酰胺
N,N-二甲基-4-(3-甲基-1,3-噁唑烷-2-基)苯胺
7-羟基-6-甲氧基苯酞
7-羟基-5-甲氧基-4-甲基苯酞
7-羟基-5-甲氧基-3H-2-苯并呋喃-1-酮
7-羟基-3H-2-苯并呋喃-1-酮
7-硝基-1(3h)-异苯并呋喃酮
7-甲氧基苯酞
7-甲氧基-8-氧杂三环[4.3.0.07,9]壬-1,3,5-三烯
7-甲氧基-6-甲基-1-氧代-1,3-二氢-异苯并呋喃-4-甲醛
7-甲氧基-4-甲基苯酞
7-甲基苯酞
7-溴-4-甲氧基苯酞
7-溴-3H-2-苯并呋喃-1-酮